INTRODUCTION
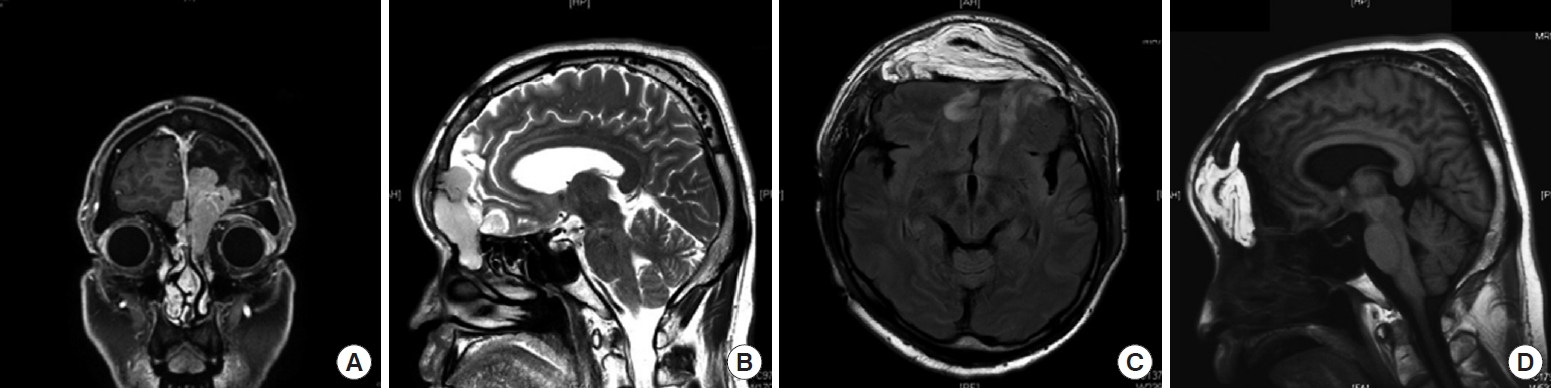
Cranial base surgery has made tremendous advancements over the past decade due to innovative developments in preoperative imaging and intraoperative monitoring. Skull base surgery, including reconstructive procedures, can be challenging even for skilled neurosurgeons because of the lack of surgical space. It is challenging for reconstructive surgeons to select the type of reconstruction that lowers the risk of major complications, such as cerebrospinal fluid leakage, meningitis, abscess, seizure, and even death [
1,
2]. Therefore, a watertight dural seal and vascularized coverage to support neural and vascular structures from the extracranial oral and nasal cavities are primary considerations.
Local flaps, distant pedicled fasciocutaneous flaps, and muscle flaps (e.g., galeal and temporalis muscle flaps) have traditionally provided vascularized tissue for dural seals [
3-
5]. The choice of a reconstructive technique depends on multiple factors (e.g., defect size and the involvement of an irradiated field). In recent years, skull base reconstruction surgery has undergone significant changes because of technological and operative advances. However, reconstruction is challenging if tumor invades the olfactory bulb, frontal sinus, or orbital wall extending from the nasal cavity to the intracranial area. In such cases, free vascularized flaps are considered the gold standard for larger defects [
4,
6]. This technique provides adequate tissue bulk as required with a reliably abundant blood supply that promotes wound healing and reduces the risk of significant postoperative complications such as cerebrospinal fluid leakage, meningitis, and pneumocephalus [
2,
4,
6,
7]. In this study, we successfully reconstructed large anterior cranial base defects that involve the intracranial and nasal cavity using the anterolateral thigh (ALT) free flap. Therefore, we suggest that an ALT free flap is a suitable and versatile surgical option to reconstruct large anterior and inner cranial base defects.
DISCUSSION
The anterior skull base, comprising the base of the anterior cranial fossa, is close to the paranasal sinuses and nasopharynx, which can be a source of infection. Its narrow and deep space and the thin basal dura tightly attached to the bone make reconstruction of the skull base challenging. In an ideal skull base reconstruction procedure, a reconstructive surgeon should conduct watertight sealing (to prevent cerebrospinal fluid leakage), separate the nasocranial spaces to secure a sufficient volume to fill the dead space, and use well-vascularized tissue to cover the defect [
9-
11].
The last decade has witnessed the emergence of new options for anterior skull base reconstruction due to improvements in microvascular techniques [
12], which has made it possible to perform more radical tumor extirpation. However, failure of anterior skull base reconstruction can cause major complications such as fatal infections due to nasocranial communication, cerebrospinal fluid leakage, tension pneumocephalus, seizure, and meningocele [
12-
14]. For small or moderate size defects local or regional flaps, such as pericranial flaps, are usually satisfactory [
15-
17]. However, to reconstruct more extensive anterior skull base defects and prevent intracranial-nasopharyngeal communication through a watertight seal, a reliable flap with named vascular supply (e.g., a bilateral reverse temporalis muscle flap) is considered an excellent choice. This flap is frequently used because it has a low incidence of failure due to the absence of vessel anastomosis and induces minimal donor site morbidity. Additionally, because the bilateral temporalis muscle is connected to the intracranial area, this flap does not require specific fixation [
18,
19]. Our institution used a reverse temporalis muscle flap for the reconstruction of anterior skull base defects. To reinforce the skull base, we additionally raised a galeal flap combined with a reverse temporalis muscle flap [
19].
Despite the positive outcomes described above, this local technique has some drawbacks: (1) local flaps, such as the pericranial flap and reverse temporalis flap, cannot reliably separate and seal the brain from the bacterial flora of the upper airway in patients with previous high-dose radiation therapy or requiring extensive resection of the cranial base [
2]; (2) osteotomy must be performed on both sides of the frontal bone to place a bilateral reverse temporalis muscle flap in the intracranial area, but this procedure provokes aesthetic complaints (e.g., depression or temporal hollowing); and (3) and prevention of intracranial complications with local flaps compared to the free flaps have shown inferior result, which may be due to the distal portion of the local flap that is placed at the most critical portion of the reconstruction having the most precarious blood supply [
20].
Therefore, our institution has recently selected free tissue transfer as the treatment of choice in these cases, carefully considering patientsŌĆÖ circumstances (e.g., previous radiation therapy or plans for postoperative radiotherapy). The choice of free flap may vary depending on the surgeonŌĆÖs preference: the flap should contain as much soft tissue as possible with a long pedicle, and free flaps are preferred for the treatment of irradiated defects [
19]. Many donor sites have been described in the literature [
21,
22], including the ALT, rectus abdominis myocutaneous, and latissimus dorsi free flaps.
A rectus abdominis free flap can be harvested without changing the patientŌĆÖs position. This flap is also advantageous because it is a myofascial free flap with substantial volume, based on the deep inferior epigastric artery, a sufficiently long pedicle. However, this approach also has limitations, such as the risk of hernia at the donor site, muscle atrophy after reconstruction, significant postoperative pain, and variation in the harvested volume due to adipose tissue [
1].
The next most commonly used flap is the latissimus dorsi free flap, which provides sufficient reconstruction volume and pedicle length. However, it also has disadvantages such as the inability to raise the flap while performing a tumor resection, muscle atrophy leading to dead space in the recipient site, and donor site morbidity. After comparing these various methods, we selected the ALT free flap as the first choice for reconstruction. The ALT flap is an excellent option for reconstructing anterior skull base defects due to its versatility, pedicle reliability, and minimal donor site morbidity without requiring an intraoperative position change [
23]. To avoid muscle atrophy, we harvested the ALT free flap mainly with fat tissue.
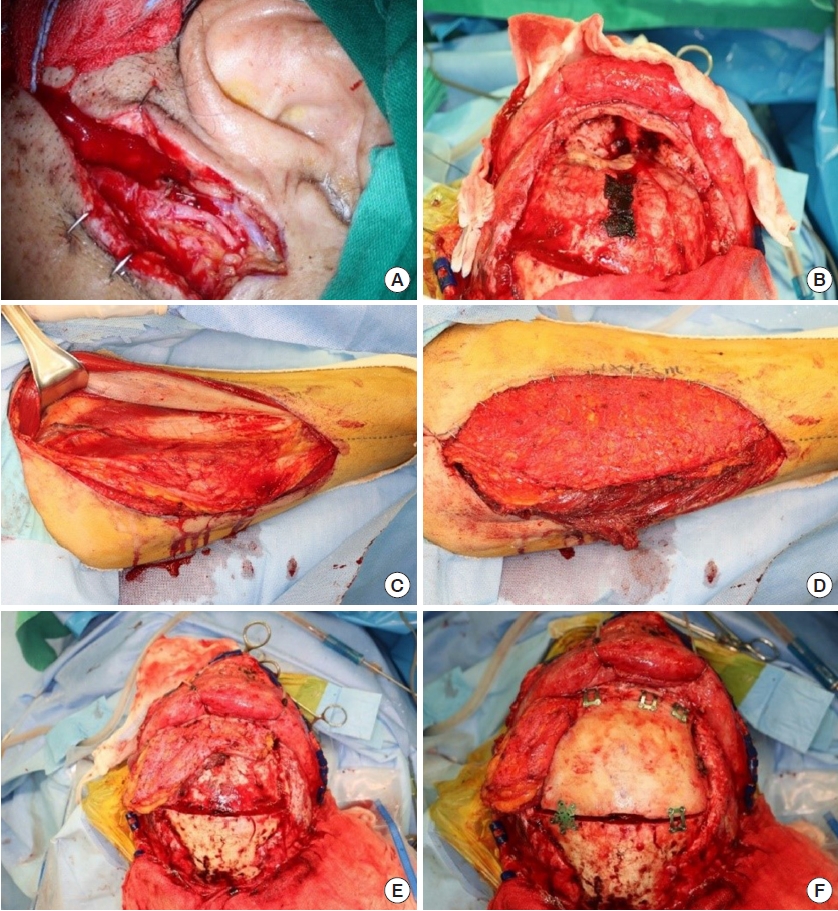
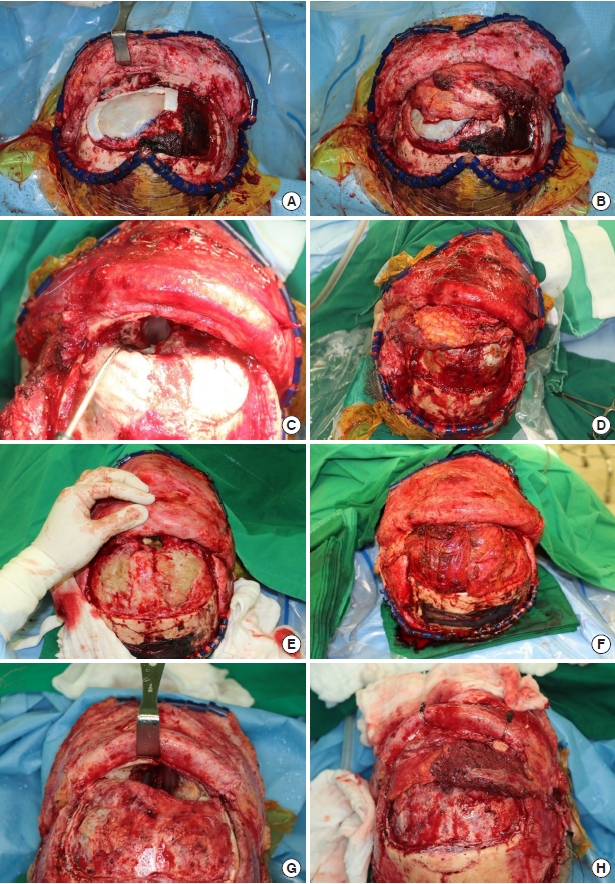
Some muscle tissue was included along the perforator during pedicle dissection. Although we did not include much muscle tissue to fill the volume, a watertight seal was well secured postoperatively with the harvested volume. Thorough de-epithelialization was performed with the dermal plexus intact because it was impossible to specify the exact location of flap fixation in advance. ALT flap elevation was performed simultaneously with tumor excision, without altering the patientŌĆÖs position. Once the defect size was determined after tumor excision, the other half of the flap outline was redesigned and further dissected. This strategy saves time and makes it easier for surgeons to maintain their focus.
The margin of the bone defect should be trimmed by burring the bony spurs to prevent irritation and the creation of dead space after insetting the flap, instead of immediately placing the flap after completing the tumor resection. Furthermore, the position where the pedicle will be placed should be refined with burring to avoid kinking and pressure after closing the scalp. After trimming the space, massive irrigation should be performed using Yankauer suction, including the nasal cavity, to prevent ascending infection. The procedure is then followed by bone fixation, Hemovac line tip, and scalp suture. A surgeon who specializes in reconstructive surgery should also perform these procedures if a neurosurgeon has not been working with the surgical team for a long time.
The dermal side is usually considered resistant to infection, but it has not been established whether to reconstruct the nasal side with the fascial side or the de-epithelized dermal side, and we did not specify which surface would face the nasal cavity. The flap surface facing the open nasal cavity was expected to undergo secondary healing; to promote this, we administered antihistamines and encouraged postoperative head elevation.
Intraoperatively managing each surgical field is critical; the vascular pedicle should be checked during neurosurgery, and the brain parenchyma should be monitored during plastic surgery In particular, these areas should be maintained in a wet environment to prevent desiccation, and irrigation and consistent monitoring are necessary. It is also recommended to insert an L-tube preoperatively to avoid misdirection toward the intracranial area if the nasal approach is not required. If the preinserted L-tube is likely to be disrupted during otorhinolaryngologic surgery, it is acceptable to insert it immediately before reconstruction.
After surgery, ascending infection of the nasal cavity should be prevented. To avoid this complication and create an environment favorable for drying, we used a continuous irrigation and suction system that was usually kept in place for 7 days postoperatively. The headŌĆÖs elevation should be maintained at 30┬░ to prevent any postoperative discharge from ascending.
In this study, successful reconstruction was performed using ALT free flaps for large anterior skull base defects or when a reverse temporalis muscle flap could not be used. The reverse temporalis muscle flap is also a good choice, particularly if technical and psychological difficulties hinder free vascularized flap transfer. A prerequisite for performing this procedure was to form a reconstructive surgery team with experience in free flaps. If reconstruction using the reverse temporalis muscle flap fails, conversion to a free flap using the proximal part of the STA and STV is the second option. However, it should be noted that if the free flap is attempted first and fails, it is possible to perform anastomosis using the STA and STV on the contralateral side or the facial artery and vein. However, this may require a longer pedicle or additional vein grafts. Considering these complications, successfully performing the first free flap is critical, while thorough preparation for the second option should be considered in advance.