INTRODUCTION
Gangrenous stomatitis has generally been thought of as associated with infection by Fusobacterium species, Prevotella species, and similar agents [1,2]. It is known to develop in children with poor nutritional status in developing countries and causes severe soft tissue deformities of the face and facial bones [3-6]. However, some cases of gangrenous stomatitis similar to noma have been reported in adult patients without malnutrition or previous bacterial infection [7,8]. Most of these patients had an immunocompromised condition such as diabetes mellitus or acquired immunodeficiency syndrome. The authors treated a case of gangrenous stomatitis caused by an opportunistic fungal infection in a patient with diabetes mellitus. This presentation is a review of the treatment process in a patient with gangrenous stomatitis using antifungal medication, surgical debridement, and staged reconstruction.
CASE REPORT
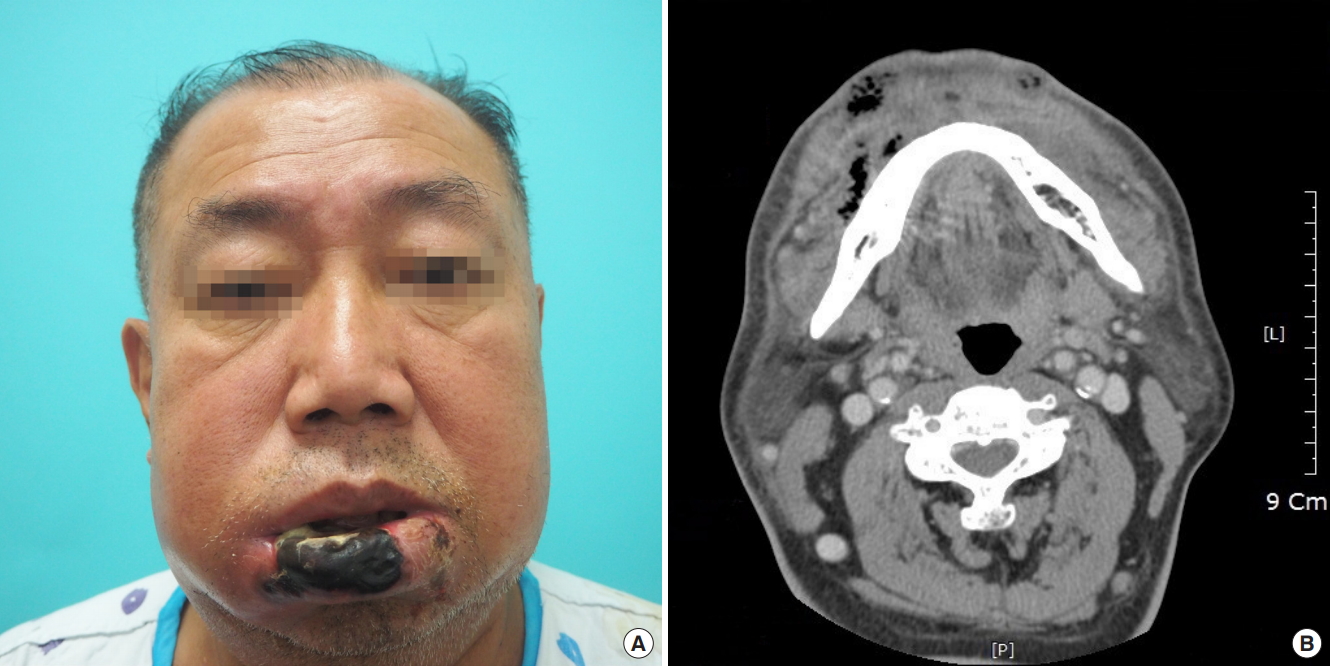
A 70-year-old male with a history of diabetes mellitus, hypertension, and coronary stent insertion visited Myongji Hospital 7 days after biting his lower lip. Swelling and inflammation had worsened, and several ulcerative mucosal lesions and purulent discharge with foul odor were observed on the lower lip. The patient was admitted for high-dose intravenous antibiotic treatment and serial debridement following demarcation of the wound.
At the time of admission, the patient had a serum glucose level of 527 mg/dL, hemoglobin A1C of 11.1%, white blood cell count of 11,000/μL, and C-reactive protein level of 9.14 mg/L. We used a beta-lactamase resistant penicillin and a quinolone as intravenous antibiotics and irrigated the oral cavity with povidone iodine and benzethonium chloride solution three to four times a day. On the 2nd hospital day, the patient’s lower lip appeared gangrenous, and the C-reactive protein level was 18.67 mg/dL. Facial computed tomography showed diffuse cellulitis with gangrenous changes and abscess formation involving the lower lip (Fig. 1). Surgical debridement was performed under local anesthesia on the 4th hospital day. Debridement involved 3/4 of the lower lip vermilion, extending to a 2.5×1.5 cm2 area of skin at the right corner of the mouth. Subsequent chemical ablation was performed on the raw surface of the debridement site using Albothyl solution, and a silastic drain was inserted. After the 1st surgical debridement, the C-reactive protein level decreased to 6.48 mg/L on the 5th hospital day. However, the wound infection and inflammatory markers did not improve. More soft tissue of the lower lip became necrotic despite surgical debridement and continued antibiotic treatment.
On the 8th hospital day, fungal infection due to Candida albicans and superimposed bacterial infection due to Klebsiella pneumoniae were found on tissue culture performed at the time of admission. An antifungal drug (fluconazole 100 mg once a day) was added and the antibiotics were changed from ampicillin/sulbactam 3 g four times a day and clindamycin 150 mg four times a day to piperacillin/tazobactam 4.5 g three times a day. On the 11th hospital day, the C-reactive protein level was 0.75 mg/dL, and signs of infection were much improved (Table 1). The decision was made to perform local flap coverage with surgical debridement. Under local anesthesia, vermilionectomy was performed on the lower lip, which had remaining debris. The defect was reconstructed using a local buccal mucosal flap. After the 2nd surgical debridement, we continued antibiotic and antifungal treatment and conservative wound dressings for 2 weeks. The C-reactive protein level gradually improved from 0.75 mg/L to 0.06 mg/L. The gangrenous stomatitis was healed by the 23rd hospital day.
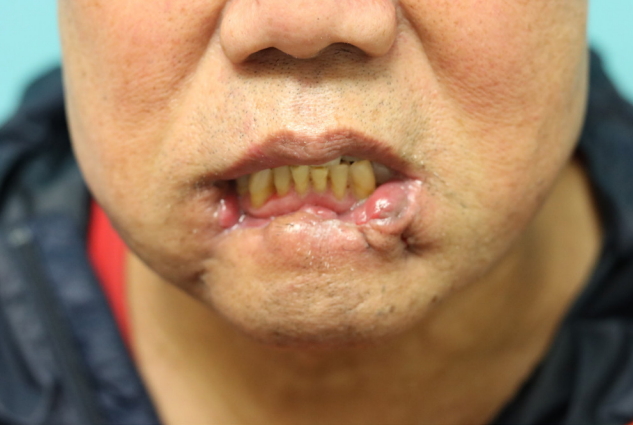
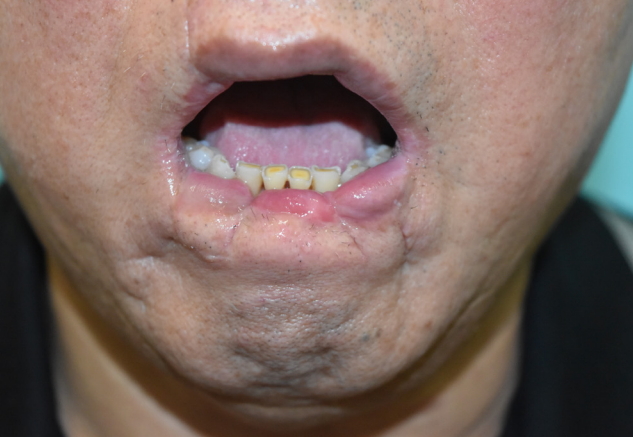
The patient was followed for about 6 months. Extensive necrosis involved approximately 3/4 of the entire lower lip, with a full-layer skin defect extending from the vermilion to the gingivobuccal sulcus at the right corner of the mouth. Because of extensive soft tissue destruction of the lower lip, the patient had functional and aesthetic defects, with trismus, incomplete mouth closure, persistent drooling, discontinuity of the lower lip, and tooth exposure (Fig. 2).
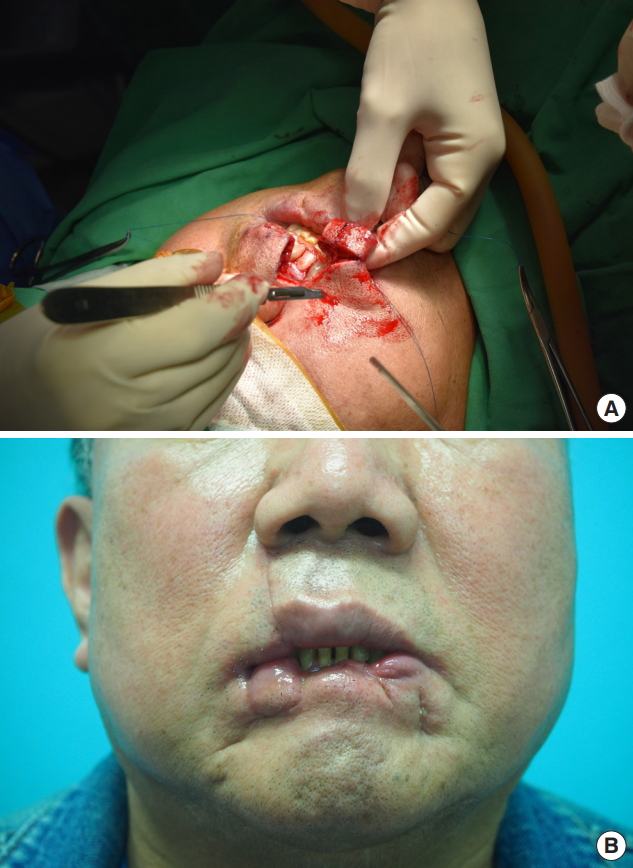
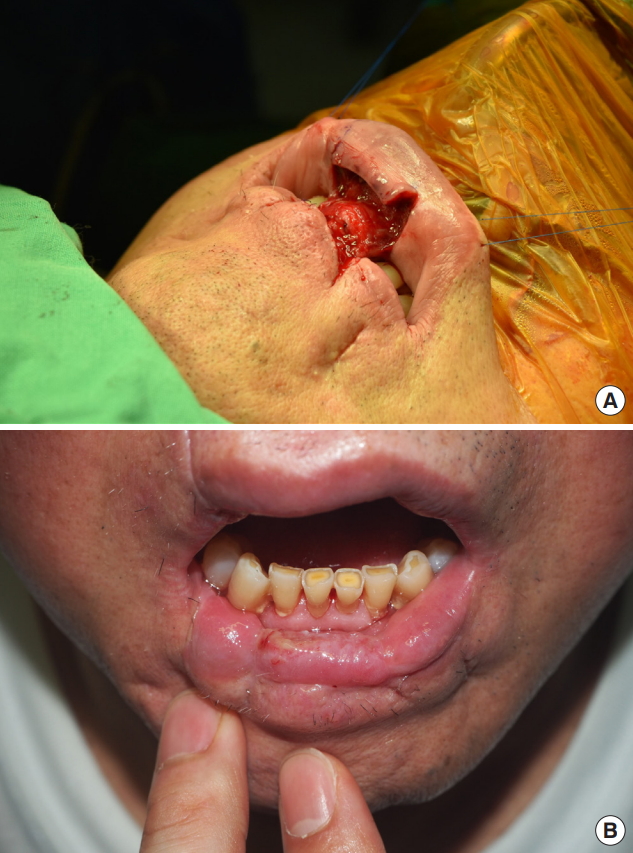
Six months later, Abbe flap coverage of the right corner of the mouth was performed to correct the drooling and trismus (Fig. 3). Three months after Abbe flap coverage, vermilion apron flap coverage of the central portion of the lower lip was performed to correct the drooling and strengthen lip sealing (Fig. 4). On the follow-up at the outpatient department, drooling from the lower lip was functionally corrected, and the patient was satisfied with the cosmetic outcome (Fig. 5). Revision of the notching deformity and incisional scars of the lower lip was planned for a later time (Table 2).
DISCUSSION
Gangrenous stomatitis is an infection of the oral cavity mainly caused by Fusobacterium species and Prevotella species [1,2]. These bacteria are pathogens involved in necrotizing stomatitis in young adults. They promote tissue destruction through their ability to degrade lipids and produce proteolytic enzymes [6]. Malnourished patients have different flora from those of healthy persons, as reported by Sawyer and Nwoku [9]. The outcome of infection has been strikingly improved by the use of antibiotics, especially penicillin, and the mortality rate is now 8%–10% [4].
Gangrenous stomatitis is almost exclusively seen in young children living in remote areas of less developed countries, particularly in Africa. However, rare cases have been reported in adult patients from developed countries, especially in the presence of blood dyscrasias and immunocompromised states [10]. Stassen et al. [7] reported the unusual presentation of necrotizing stomatitis in a healthy white adult Caucasian woman in a part of the world where the disease is very uncommon. Moon et al. [8] reported facial deformities following necrotizing stomatitis in five patients aged between 47 and 58 years.
Our patient was a 70-year-old man with diabetes mellitus, hypertension, and coronary stent insertion. Patients with diabetes mellitus are in an immunocompromised state, as a result of two mechanisms. First, diabetic polymorphonuclear cells and monocytes/macrophages have decreased function (chemotaxis, phagocytosis, killing), and second, microorganisms show increased adherence to diabetic cells. C. albicans expresses a surface protein that has great homology with the receptor for complement factor 3b. In a hyperglycemic condition, expression of the receptor-like protein of C. albicans is increased, resulting in competitive binding and inhibition of complement-mediated phagocytosis [11]. Darwazeh et al. [12] recently showed a significant increase in the adhesion of Candida to oral epithelial cells in patients with diabetes, when compared with a healthy population.
Adhesion of Candida to epithelial cells, widely recognized as an essential step in the process of Candida colonization and subsequent infection, is significantly inhibited by fluconazole. Fluconazole is secreted in the saliva in high concentration. It is tempting to speculate that fluconazole may interfere with the synthesis or structure of Candida receptors in buccal epithelial cells. Candida colonizes mucocutaneous surfaces, which can be portals of entry into deeper tissues when host defense is compromised [11].
In our case, a small wound developed in the oral mucosa and Candida present in the patient’s mouth may have infected deep soft tissue, resulting in extensive soft tissue injury. Early detection of fungal infection prior to wound culture and the early use of fluconazole might have reduced the extensive soft tissue injury. Furthermore, the outcome would have been better if the patient had visited the hospital earlier, before worsening of the infection. Further studies are needed to investigate the prevalence of stomatitis caused by oral fungal infections in patients with diabetes, as well as the efficacy of prophylactic antifungal agents.
This report described a case of gangrenous stomatitis due to a fungal opportunistic infection in a patient with diabetes mellitus. The resulting extensive soft tissue defect extending from the vermilion to the gingivobuccal sulcus caused drooling and trismus. Patients with diabetes mellitus are in an immunocompromised state, and should maintain oral hygiene, control blood glucose levels, and receive appropriate and timely wound care for even small oral lesions. Physicians should always be mindful of the possibility of oral fungal infections in patients with diabetes mellitus and should always treat these as high-risk oral infections.