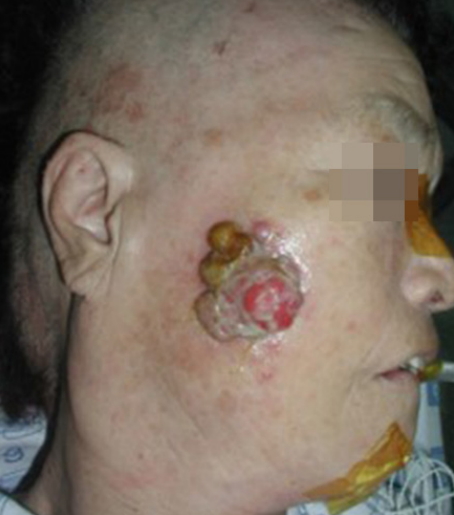
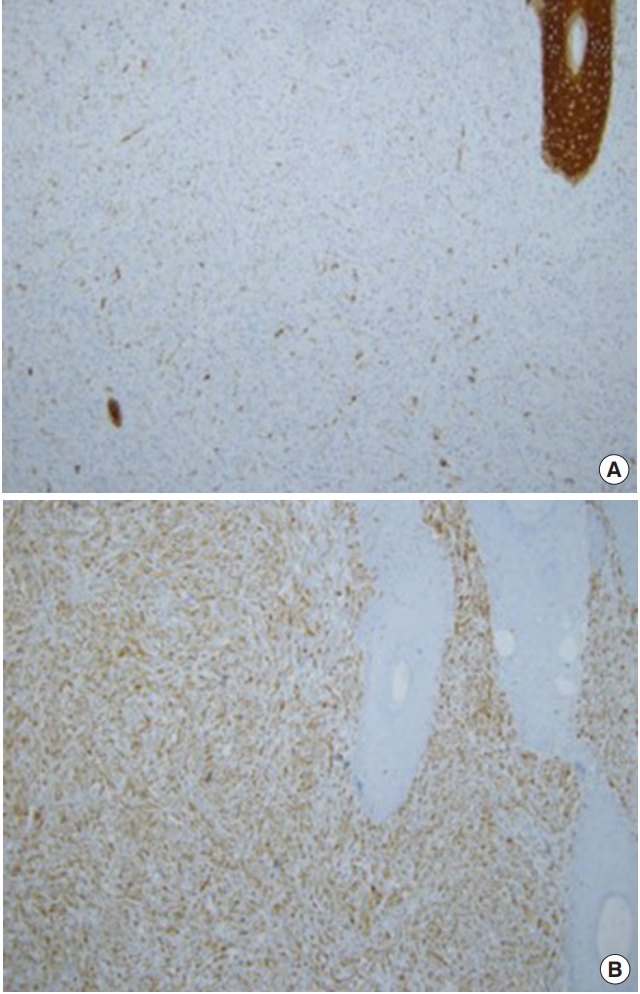
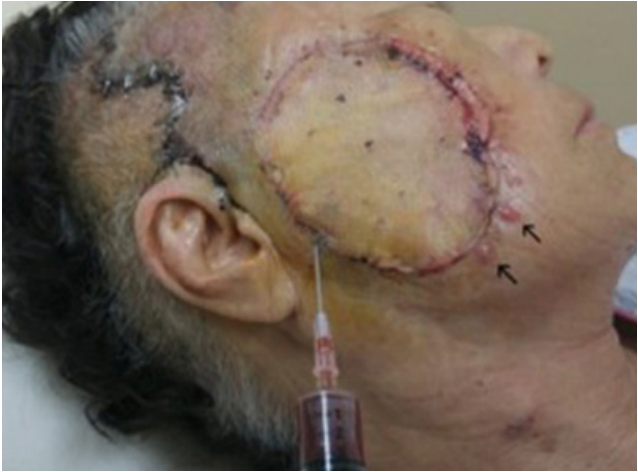
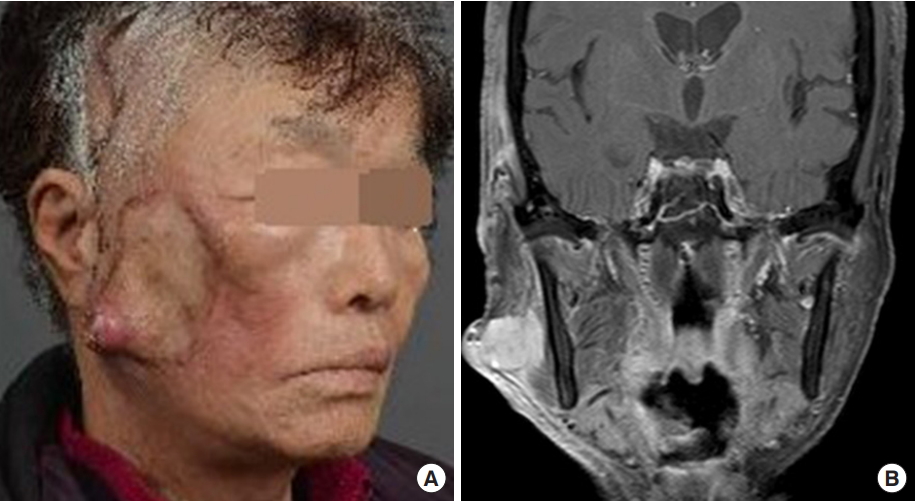
1. Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K. FitzpatrickŌĆÖs dermatology in general medicine. 8th ed. New York: McGraw-Hill; 2012.
3. Kim YD, Suhr KB, Lee JH, Park JK. A clinical and histopathological study of cutaneous squamous cell carcinoma. Korean J Dermatol 2002;40:25-30.
4. Chi DH, Sung KJ, Koh JK. A clinical observation of primary epithelial skin cancers. Korean J Dermatol 1995;33:1085-90.
5. Hayes AJ, Clark MA, Harries M, Thomas JM. Management of in-transit metastases from cutaneous malignant melanoma. Br J Surg 2004;91:673-82.


8. Ma JH, Wu A, Veness M, Estall V, Hong A, Borg M, et al. Intransit metastasis from squamous cell carcinoma. Dermatol Surg 2016;42:1285-92.


10. Carucci JA, Martinez JC, Zeitouni NC, Christenson L, Coldiron B, Zweibel S, et al. In-transit metastasis from primary cutaneous squamous cell carcinoma in organ transplant recipients and nonimmunosuppressed patients: clinical characteristics, management, and outcome in a series of 21 patients. Dermatol Surg 2004;30:651-5.


11. Copcu E, Dikicioglu E, Sivrioglu N, Meteoglu I. Subcutaneous nodules on the face: acantholytic in-transit cutaneous metastasis. Dermatol Surg 2004;30:1415-9.


12. Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip: implications for treatment modality selection. J Am Acad Dermatol 1992;26:976-90.


13. Lewis JE, Olsen KD, Sebo TJ. Spindle cell carcinoma of the larynx: review of 26 cases including DNA content and immunohistochemistry. Hum Pathol 1997;28:664-73.


15. Bigby SM, Charlton A, Miller MV, Zwi LJ, Oliver GF. Biphasic sarcomatoid basal cell carcinoma (carcinosarcoma): four cases with immunohistochemistry and review of the literature. J Cutan Pathol 2005;32:141-7.


16. Ribatti D. Epithelial-mesenchymal transition in morphogenesis, cancer progression and angiogenesis. Exp Cell Res 2017;353:1-5.


18. Folpe AL, Cooper K. Best practices in diagnostic immunohistochemistry: pleomorphic cutaneous spindle cell tumors. Arch Pathol Lab Med 2007;131:1517-24.


19. Lydiatt WM, Patel SG, OŌĆÖSullivan B, Brandwein MS, Ridge JA, Migliacci JC, et al. Head and neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:122-37.