Lymphaticovenular anastomosis for Morbihan disease: a case report
Article information
Abstract
Morbihan disease (MD) is a very rare condition characterized by rosaceous or erythematous lymphedema on the upper twothirds of the face. A definitive management strategy for MD is lacking, and treatment is challenging. Herein, we present a case of persistent bilateral eyelid edema treated by lymphaticovenular anastomosis (LVA) and lymph node-vein bypass surgery. The patient experienced persistent bilateral eyelid edema. Indocyanine green lymphography was performed, and the diagnosis of bilateral facial lymphedema was confirmed. On the right side, a preauricular lymphatic vessel was anastomosed to a vein. On the left side, lymphostomy on the preauricular lymph node was done, with anastomosis to the transected proximal end of the concomitant vein of the transverse facial artery. Furthermore, a preauricular lymphatic vessel was anastomosed to a vein. Eyelid edema decreased and progressively improved on both sides. The outcome of this case suggests that LVA and lymph node-vein bypass surgery are appropriate for treating persistent eyelid edema related to MD.
INTRODUCTION
Morbihan disease (MD) was first reported in 1957 by Robert Degos. MD involves rosaceous lymphedema, with chronic erythematous edema in the upper two-thirds of the face. It often affects the eyelids, forehead, glabella, and cheeks. MD is a very rare syndrome that mostly affects Caucasians [1-3]. Because MD has similar symptoms to those of many granulomatous and inflammatory diseases, it is difficult to diagnose. The differential diagnosis of MD includes systemic lupus erythematosus, cutaneous leishmaniasis, foreign body granuloma, facial granuloma, superior vena cava syndrome, rhinophymas and scleredema of Buschke, as well as reactions to drugs such as barbiturates, chlorpromazine, diltiazem, and isotretinoin [4-7]. Since persistent and severe facial edema can cause visual impairment, it is vitally important to treat this condition [1]. However, there is no definitive treatment for MD. Previous studies have described using medications, including systemic steroids, tetracycline, antibiotics (e.g., doxycycline), thalidomide, isotretinoin, and topical regimens. Nonetheless, MD is often refractory to pharmacological therapy [3,8]. If pharmacological therapy does not work, eyelid reduction surgery, lymphatic drainage surgery, carbon dioxide laser treatment, and local steroid injections can be considered [3,9,10]. Partial excision is inefficient because recurrence is likely, whereas total excision is not suitable from a functional and aesthetic standpoint. To resolve this dilemma, the use of lymphaticovenular anastomosis (LVA) has been described. However, LVA is not yet recognized as a definitive treatment modality for MD due to the insufficient number of published cases.
The purpose of this study is to report a case of MD that recovered well, without recurrence, through LVA and lymph node-vein bypass surgery. This report may provide a basis for recognizing LVA as a suitable option for the treatment of persistent eyelid edema in patients with MD.
CASE REPORT
A 64-year-old man experienced persistent bilateral eyelid edema with no specific history. The patient had undergone excisional surgery on the lower right eyelid at the Department of Ophthalmology 12 years previously, but the condition recurred again (Fig. 1) [3]. At the Department of Rehabilitation Medicine, a treatment regimen of complex lymphatic physiotherapy and micronized flavonoids (Venitol, Kwangdong Pharm.) was applied for 3 months, but to no effect. Thus, the patient was referred to attempt surgical treatment. Indocyanine green (ICG) lymphography was performed, showing severe dermal back flow in the bilateral periorbital region. The diagnosis of bilateral facial lymphedema was confirmed (Fig. 2) [3]. We decided to perform LVA and lymph node-vein bypass surgery first under local anesthesia. Preoperatively, mapping of the lymphatic vessels was performed through ICG lymphography. After ICG dye injection, images of the lymphatic drainage were obtained using a near-infrared camera (Moment K; IANC&S). Indigo carmine (blue dye) was then injected in the surgical field and the lymphatic vessels were visualized. During surgery, we found a branch of the superficial temporal vein that matched the size of the corresponding lymphatic vessel. On the right side, a preauricular lymphatic vessel (0.3 mm) was anastomosed to a vein (0.4 mm) in an end-to-end manner. On the left side, lymphostomy of the preauricular lymph node was done, followed by anastomosis to the transected proximal end of the concomitant vein (1.6 mm) of the transverse facial artery. A preauricular lymphatic vessel (0.3 mm) was also anastomosed to a vein (0.6 mm) in an end-to-end manner (Fig. 3). Immediately after surgery, periorbital edema diminished, and progressive improvement was observed in the bilateral periorbital region (Fig. 4).
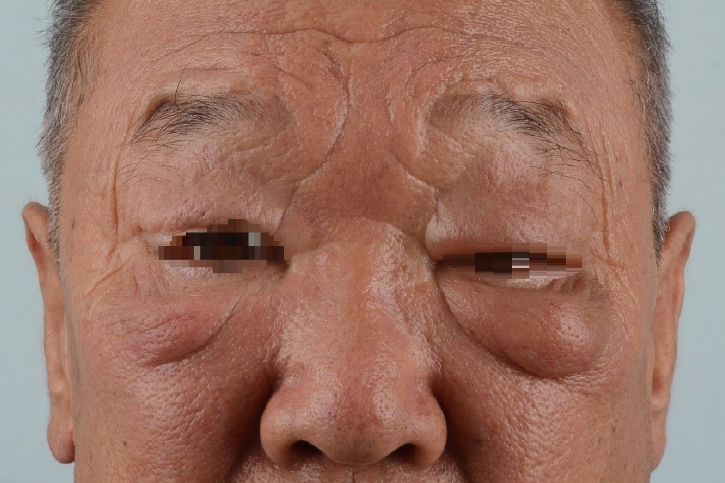
Preoperative appearance of a 64-year-old male patient with chronic, non-pitting edema in the periorbital region.
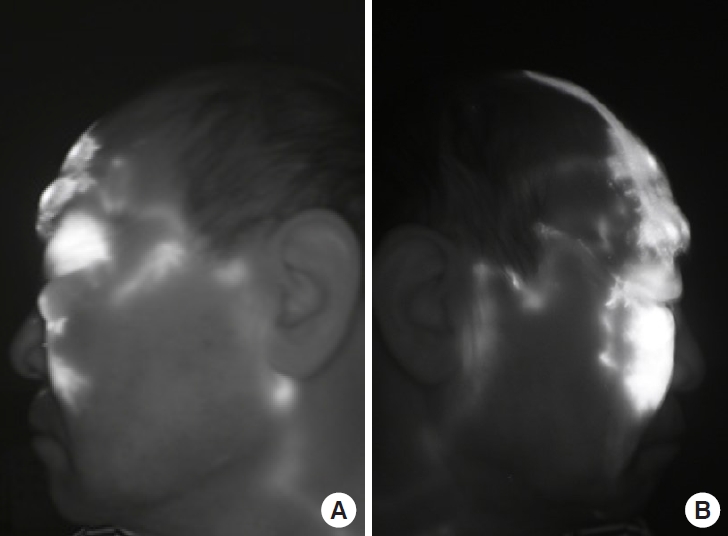
Indocyanine green lymphography. (A) Left side. (B) Right side. Severe dermal backflow is noted in both (A) and (B). Patent lymphatics of both preauricular regions were identified.
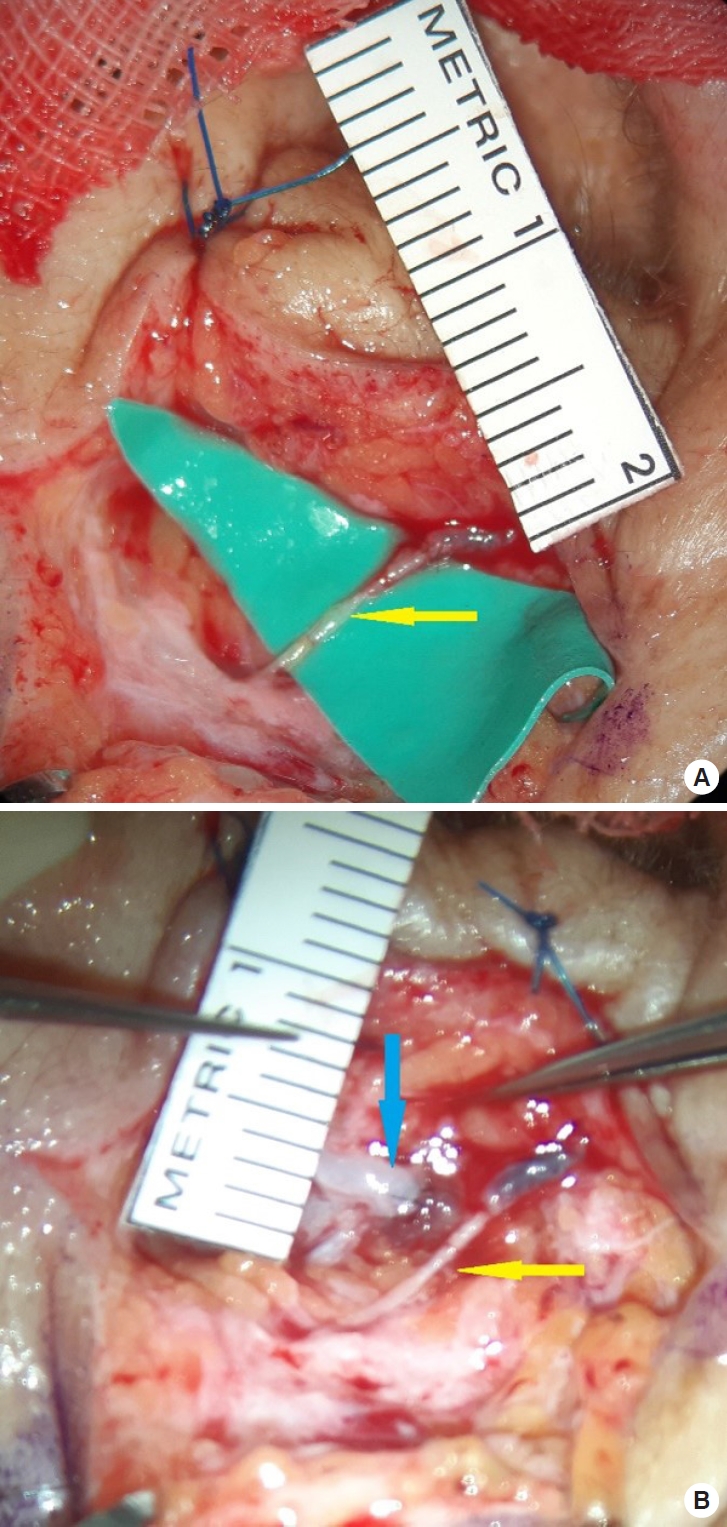
Intraoperative images. (A) A right preauricular lymphatic vessel (0.3 mm) was anastomosed to a vein (0.4 mm) in an end-toend manner (yellow arrow). (B) Left side. Lymphostomy of the preauricular lymph node was done, and it was anastomosed to the transected proximal end of the concomitant vein (1.6 mm) of the transverse facial artery (blue arrow). Additionally, a preauricular lymphatic vessel (0.3 mm) was anastomosed to a vein (0.6 mm) in an end-to-end manner (yellow arrow).
At 3 years and 5 months after surgery, the patient was still very satisfied.
DISCUSSION
MD, also called rosaceous lymphoedema, is clinically characterized by firm, non-pitting edema and erythema. Symptoms appear throughout the middle and upper thirds of the face [4,8,11]. The pathogenesis of MD is unclear. However, it is known that chronic inflammation damages the lymphatic vessel walls and alters lymphatic drainage, resulting in persistent lymphedema. The histopathologic findings suggest dilation of lymphatic vessels, non-caseating granulomas, mast cell infiltration to the periadnexal tissues of the lymph vessels, and sebaceous gland hyperplasia [3,11].
The diagnosis of MD is challenging because there are no distinctive histopathological or biochemical findings. Therefore, it is necessary to differentiate MD from other diseases, and a biopsy in patients with clinical symptoms is recommended. The patient in this case had non-pitting edema in the periorbital region for the past 16 years. In addition, preoperative ICG lymphography confirmed severe bilateral dermal backflow.
The choice of a treatment method for MD requires careful consideration, since there is no single definitive treatment method. Pharmacological treatment is possible, but systemic steroids and antibiotics such as tetracycline require a lengthy course of treatment (at least 4 to 6 months), potentially causing adverse effects, and there is still no consensus regarding the treatment method due to a lack of research on usage [4,11,12]. During the excision extensive surgery may deform the normal shape and has a high probability of recurrence. The patient presented herein had previously undergone excision surgery at an ophthalmology clinic, but the condition recurred. Recurrence has often been reported, and simple resection is a method that causes long-term pain, as well as being socially and economically unfavorable [13]. LVA was first developed by Yamada [14] and O’Brien et al. [15,16]. However, recent technological advances in LVA and supermicrosurgery have been developed by Koshima et al. [17], and numerous studies support LVA as firstline therapy in lymphedema patients. Numerous papers have demonstrated LVA to be effective in the upper and lower extremities. LVA involves creating a new lymphatic vein bypass. Therefore, lymphatic drainage surgery is a sufficiently effective method for treating MD [3]. One option is to perform lymph node transfer and lymphatic channel transfer, but those methods involve a long operation time and are difficult to perform under local anesthesia. Furthermore, from an aesthetic standpoint, those methods are not appropriate. In this case, we connected the lymphatic collecting vessel of each bilateral preauricular region with the corresponding vein, and performed nodalvenous anastomosis on the left side. On the left side, where functional collecting lymphatic vessels could not be identified, it was necessary to perform surgery with proper lymph nodevein bypass. Compression can be performed after LVA surgery in the extremity. A previous report stated that the venous pressure is higher than the lymphatic pressure before compression, causing backflow to occur. Compression increases the lymphatic pressure due to the presence of outflow obstruction associated with lymphedema, resulting in a reversal of flow and the washout sign [18]. However, unlike in the extremities, compression is not possible in the facial area. We perform lymph node-vein bypass surgery when (1) no functional lymphatic collecting vessel can be identified and (2) when sufficient lymphatic fluid is observed after lymphostomy. Through these two methods, the outflow of lymph drainage in the periorbital region was increased, ensuring effective drainage [3].
In conclusion, a patient was diagnosed with MD through ICG lymphography, and satisfactory results were obtained without recurrence through LVA and lymph node-vein bypass surgery. The above surgical method is simple and can be performed under local anesthesia. This surgical approach may be helpful for the treatment of MD in the future.
Abbreviations
ICG
indocyanine green
LVA
lymphaticovenular anastomosis
MD
Morbihan disease
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
No potential conflict of interest relevant to this article was reported.
Ethical approval
This report was approved by the Institutional Review Board of Pusan National University Hospital (IRB No. 2211-008-120).
Patient consent
The patient provided written informed consent for the publication and the use of his images.
Author contributions
Conceptualization: Yong Chan Bae, Joo Hyoung Kim. Formal analysis: Ryuck Seong Kim. Funding acquisition: Joo Hyoung Kim. Methodology: Yong Chan Bae. Project administration: Jae Woo Lee, Joo Hyoung Kim. Writing - original draft: Jung Hyun Hong. Writing - review & editing: Ryuck Seong Kim, Joo Hyoung Kim. Investigation: Jung Hyun Hong. Supervision: Changryul Claud Yi, Jae Woo Lee. Validation: Changryul Claud Yi.

