 |
 |
- Search
| Arch Craniofac Surg > Volume 24(5); 2023 > Article |
|
Abstract
Angiosarcoma is a very rare soft tissue sarcoma that originates from endothelial cells and typically has a poor prognosis. It is most commonly found in elderly white men and can occur anywhere in the body, particularly in the head, neck, and scalp. Patients who have undergone previous radiation treatment or who have chronic lymphedema also face an elevated risk of this condition. Various genetic changes are suspected to contribute to the development of angiosarcoma, and these changes have been identified as potential targets for treatment. For localized disease, wide surgical resection is often the prudent course of action. A multidisciplinary approach, which may include surgery, radiotherapy, systemic chemotherapy, or immunotherapy, is typically the most effective way to achieve favorable outcomes. In this review, we discuss the general understanding of angiosarcoma and its management, with a particular focus on the current evolving treatments for the disease.
Angiosarcomas are soft tissue sarcomas that originate from endothelial cells. They are exceedingly rare, accounting for less than 1% of all sarcomas [1]. These tumors can develop anywhere in the body and can occur at any age. However, they most commonly present as cutaneous disease in elderly white men, particularly affecting the head and neck region, especially the scalp. Angiosarcomas can also originate from the breast, soft tissues, bone, and visceral organs [2,3]. Several risk factors for angiosarcomas have been identified, including therapeutic radiation, chronic lymphedema, exposure to various carcinogens, and several genetic syndromes [4,5]. Treatment options include surgical resection for localized disease and systemic chemotherapy for metastatic disease. Achieving negative margins can be challenging due to the infiltrative nature of these tumors. A multidisciplinary approach, which may include surgical resection, chemotherapy, radiotherapy, or potential immune-agents, could yield positive outcomes [4-6]. In this article, we aim to review the fundamental characteristics of angiosarcomas and provide a brief overview of the up-to-date treatment options for this disease.
Angiosarcomas account for up to 2% of all soft tissue sarcomas and 5.4% of cutaneous soft tissue sarcomas [7,8]. These tumors are more prevalent among the elderly, with the average age of patients being 73, and they exhibit a similar incidence rate in both sexes [9]. However, angiosarcomas of the head and scalp are more common in males, while those associated with radiation and lymphedema are more frequently observed in females [10,11]. Despite the endothelial cell origin of these tumors, it is rare for them to arise directly from major blood vessels or the heart. Instead, they can develop in any soft tissue structure or viscera [3,12].
Angiosarcomas can be categorized into five types: cutaneous angiosarcoma, lymphedema-associated angiosarcoma, radiation-induced angiosarcoma, primary-breast angiosarcoma, and soft tissue angiosarcoma [13]. Several risk factors have been well described in the literature (Table 1). Chronic lymphedema is a well-recognized factor in the development of breast angiosarcomas following breast cancer treatment. Similar conditions, such as Milroy disease, chronic infections, and filariasis, have also been associated with the development of angiosarcomas [4,14]. Likewise, radiotherapy is acknowledged as an independent risk factor for angiosarcomas, although the connection between radiotherapy and subsequent angiosarcoma is disputed, with some suggesting that the risk may stem from concurrent lymphedema [10,11,15-17]. Various familial syndromes, including neurofibromatosis, Maffucci syndrome, and Klippel-Trenaunay syndrome, have been linked to angiosarcoma. Additionally, some case reports have identified mutations in BRCA1 and BRCA2 as potential risk factors for angiosarcomas following breast cancer treatment [18]. Carcinogens such as vinyl chloride, thorotrast, androgenic steroids, phenylhydrazine, arsenic, and radium are considered potential etiological agents in visceral angiosarcoma, possibly accounting for up to 25% of cases [19,20]. Other proposed risk factors include ultraviolet radiation, immunocompromised states, arteriovenous fistulas, and xeroderma pigmentosum [21-23].
Histologically, the characteristics of angiosarcomas can vary both within a single case and between different cases. It can be challenging to distinguish the morphological features of a malignant vascular tumor from a benign lesion that exhibits proliferation. However, angiosarcomas typically present with abnormal, pleomorphic, malignant endothelial cells. These cells can take on various shapes, including rounded, polygonal, or fusiform, and often exhibit an epithelioid appearance. In areas where the tumor is well-differentiated, it may mimic hemangiomas or lymphangiomas. Here, atypical epithelial cells or diffuse epithelioid and spindle cell proliferations form functioning vascular sinusoids. As the disease becomes more aggressive, these structures grow more complex, with multilayered endothelial cell lining and the formation of papillary projections. Highgrade tumors often exhibit mitotic activity. Indicators of a poorly differentiated, high-grade tumor include necrosis and hemorrhage, along with continuous sheets of endothelial cells [4,5,23,24].
An immunochemical analysis reveals that angiosarcoma typically expresses endothelial markers such as CD31, CD34, von Willebrand factor, Ulex europaeus agglutinin 1, and vascular endothelial growth factor (VEGF). Of these markers, von Willebrand factor, Ulex europaeus agglutinin 1, and CD31 are particularly useful in distinguishing poorly differentiated diseases. Additionally, the absence of melanocytic markers, including S100, human melanoma black-45, and melanoma antigen, can aid in differentiating angiosarcoma from melanoma [25].
While no specific chromosomal abnormalities have been identified in the development of angiosarcoma, certain somatic gene mutations have been reported. Behjati et al. [26] examined 39 angiosarcoma cases and discovered somatic mutations in PTLR and PLCG1, as well as in KRAS, NRAS, HRAS, PIK3CA, and FTL4. Other research has identified mutations in TP53 and CDKN2A, which were the most frequently altered genes in the study, with half of the cases exhibiting MAPK pathway derangements [27]. In addition, several studies have shown that the amplification of the proto-oncogene MYC is commonly found in radiation-induced angiosarcoma, but not in sporadic types [15,27,28]. This broad spectrum of genetic abnormalities may account for the clinical variability of tumor subtypes. As such, the use of gene-expression microarray technology is crucial for identifying molecular markers, and a multidisciplinary approach is necessary for treatment.
Despite their aggressive nature, angiosarcomas initially present as a bruise, erythematous patch, plaque, or small papule (Fig. 1) [10,24]. Their innocuous clinical appearance often leads to delayed presentation and diagnosis [29]. As the tumor size increases, symptoms such as ulceration, hemorrhage, tissue infiltration, edema, and tumor fungation may occur. The invasion of surrounding structures can result in unclear resection margins, leading to large soft tissue defects following radical resection surgery (Fig. 2) [12,29,30]. The visceral type of angiosarcoma can occur in the spleen, liver, or even the heart. It often presents as an expanding mass associated with pain and discomfort, and rarely with pericardial effusion. Angiosarcomas may also present as pleural disease, hemorrhagic pleural effusion, or pneumothorax due to hematogenous spreading. Other common sites of metastasis include the liver, bone, skin, and regional lymph nodes [3,29,31-33].
Angiosarcomas have a 5-year survival rate that varies between 30% and 56%. Even the most optimistic study indicates that only 60% of patients with localized disease survive beyond 5 years [3,12,31,34]. While there are studies documenting longterm survivors with metastatic disease, they have not been able to identify significant prognostic factors [12]. Numerous studies have sought to uncover the prognostic factors of angiosarcomas. Similar to other aggressive tumor types, age, size, grade, and margin status have been identified as indicators of recurrence and survival [5]. Although angiosarcomas can develop in young patients and even children, it is predominantly a disease of the elderly [35]. A poor prognosis in elderly patients may be due to a longer interval between initial diagnosis and treatment, a more compromised immune system, and a poorer performance status [29]. From the standpoint of tumor biology, a tumor size exceeding 5 cm is associated with worse outcomes [24,36-41]. Tumor depth is another factor linked to a poorer prognosis, but there is no consensus on whether greater tumor depth predicts worse survival or only affects local recurrence [24,31,42]. Other factors that predict a poor prognosis include the metastatic state at presentation and poor patient performance [12,29,43]. Furthermore, the site of origin also influences the prognosis, with angiosarcomas of the liver or other viscera and retroperitoneal disease associated with a worse prognosis compared to cutaneous disease [12].
While there have been no specific randomized clinical trials for angiosarcomas, treatment has been guided by the published guidelines for other soft tissue sarcomas. There has been no evidence-based management available for patients with this rare disease. However, a multidisciplinary approach to treatment is always recommended to manage the disease and enhance survival outcomes. Currently, more clinical trials are being conducted to broaden treatment options and improve patient outcomes.
For localized disease, the standard treatment is radical surgery with a complete resection margin. However, achieving wide negative margins can often be challenging due to tissue infiltration and involvement of anatomical regions such as cardiac tumors and head and neck tumors, which render the disease unresectable [12,29-31]. Choi et al. [44] reported that only a deep margin for excision was significantly related to recurrence. However, the prognostic significance of surgical margins remains to be clarified, and surgical efforts should strive to achieve a microscopically clear margin whenever possible [44,45]. A staged approach may be employed when definitive pathologic margins are determined at the initial resection. In such cases, re-excision can be performed, followed by reconstruction [29].
Several studies have reported that non-metastatic patients who have undergone surgical resection exhibit improved recurrence and survival rates compared to those who have not been resected [28,36,39,46,47]. However, due to the absence of randomized studies, the general approach favors surgical management of angiosarcomas in non-metastatic cases when possible. Nevertheless, other treatment modalities should be considered to enhance patient outcomes [5].
Due to the high risk of local recurrence, adjuvant radiation is another method used in the management of angiosarcoma. Although no randomized trials have been conducted, several studies indicate that it enhances local control and overall survival [3,6,29,36,46]. Resection surgery combined with radiotherapy yields better results than either radiation or surgery alone, underscoring the need for a multidisciplinary approach [29,36]. However, in cases of radiation-induced angiosarcoma, radiotherapy is often avoided due to dose-related toxicity. Despite its limited use, radiotherapy should still be considered as an adjuvant measure to reduce the risk of local recurrence in angiosarcomas [5].
It is clear that there is no evidence supporting the application of neoadjuvant or adjuvant chemotherapy following resection surgery and radiotherapy for angiosarcoma [32,48-50]. However, systemic chemotherapy is the primary option in advanced and metastatic cases [4-6]. Angiosarcomas have been treated in a manner similar to other soft tissue sarcomas, using an anthracycline-based regimen. The effectiveness of this anthracyclinebased therapy in treating angiosarcoma is comparable to its effectiveness in treating other types of sarcoma. Notably, the combination of ifosfamide with anthracycline has been shown to result in a longer progression-free survival period than treatment with anthracycline alone [51].
The application of pegylated doxorubicin is typically restricted to certain types of soft tissue sarcoma, such as desmoid tumors. Numerous studies have indicated the potential efficacy of pegylated doxorubicin in treating angiosarcomas. Therefore, it could be a viable alternative for patients who are unable to withstand more aggressive chemotherapy treatments [52].
Paclitaxel is frequently employed as a first or second-line treatment for angiosarcoma due to the antiangiogenic activity of taxanes. The effectiveness of paclitaxel was evaluated in a multicenter phase II study involving 30 angiosarcoma patients. The study reported an overall response rate of 19% at 6 months. Several retrospective studies have also confirmed the efficacy of paclitaxel [53-55].
Gemcitabine combined with docetaxel is a common secondline treatment for advanced soft tissue sarcomas, yet no prospective studies exist for metastatic angiosarcoma. Recently, an alternative combination of gemcitabine with nanoparticle albumin– bound paclitaxel (nab-paclitaxel) was explored. The overall better toxicity profile of nab-paclitaxel compared to paclitaxel may offer an alternative option. Future studies should consider these combinations for use in advanced angiosarcomas [56,57].
Eribulin, an agent that disrupts microtubule polymerization, has recently been reported in cases of cutaneous angiosarcoma that have progressed with taxanes. This agent, which exhibits less neurotoxicity than taxanes, may provide a treatment option for cases that have shown progression despite treatment with taxanes and are not eligible for an anthracycline-based regimen [58].
Encequidar is an adenosine triphosphate binding cassette transporter P-gp inhibitor that prevents the efflux of cytotoxic agents from epithelioid cells to the gastrointestinal tract, thereby promoting increased oral bioavailability and enhancing the efficacy of chemotherapy. A recent phase II study investigated the combination of encequidar and oral paclitaxel for patients with unresectable cutaneous angiosarcoma who had not previously undergone taxane therapy. The study demonstrated favorable side effect profiles, particularly considering the notably advanced median age of the patients [59].
In angiosarcomas, VEGF and its receptors are overexpressed, making the targeting of this pathway a feasible option. Bevacizumab, a VEGF inhibitor, has demonstrated a modest effect in advanced angiosarcoma [60]. The combination of bevacizumab with paclitaxel, gemcitabine, or docetaxel has been tested in several studies. However, whether these combination strategies exhibit strong activity requires further large-scale prospective investigation [61,62].
Tyrosine kinase inhibitors (TKIs) possess anti-VEGF activity and have been the subject of studies in angiosarcoma. Pazopanib, a TKI approved for use in soft tissue sarcomas, has demonstrated modest benefits in retrospective studies. An antibody to endoglin, known to mediate resistance to pazopanib, was examined in a phase III trial but did not exhibit significant activity [63-66]. Regorafenib, another TKI with anti-VEGF activity, showed some effectiveness in a small number of angiosarcoma patients [67]. Propranolol, which is typically effective in treating benign vascular tumors, has been investigated and utilized in the treatment of some angiosarcoma patients [68].
Pembrolizumab, an anti-PD1 (programmed death 1) checkpoint inhibitor, has been approved for use in tumors with a high tumor mutational burden. It was previously reported that this checkpoint inhibitor was effective in treating three out of ten angiosarcoma patients who showed no response to standard therapies [69,70]. Other checkpoint inhibitors, such as anti-PD-L1 or anti-CTLA-4 agents, have also been reported to elicit varying responses in cutaneous angiosarcomas and radiation-associated breast angiosarcomas [71-73]. Current investigations are focusing on using immunotherapy alone or in combination with systemic chemotherapy, TKIs, or an oncolytic virus to treat angiosarcomas.
Isolated limb perfusion (ILP) is a procedure that involves administering chemotherapy drugs directly into a limb affected by locoregional metastasis. ILP has been explored as an alternative treatment when radical surgery aimed at preserving function is not viable. While some studies have demonstrated the effectiveness of ILP, its use can be challenging due to the risk of severe complications and a general lack of expertise in the technique [74]. For primary liver angiosarcomas, transarterial embolization has shown minimal impact on survival rates. However, intra-arterial therapies may be a viable option when it is necessary to manage liver disease [75-77].
Angiosarcoma is an extremely rare soft tissue sarcoma, characterized by its complex biology and aggressive nature. For the treatment of angiosarcoma without clear metastasis, a wide margin resection is necessary, coupled with a staged multimodal approach. The use of both radiotherapy and systemic chemotherapy is advisable to enhance outcomes, while antiangiogenic biological therapies may provide specific treatments for angiosarcoma. Further clinical trials, grounded in translational work, hold the potential to significantly influence and clarify treatment strategies.
Notes
Fig. 1.
Morphological findings of scalp angiosarcoma in (A) an 81-year-old woman and (B) an 85-year-old woman with scalp angiosarcoma resembling skin bruise, crust or plaque.
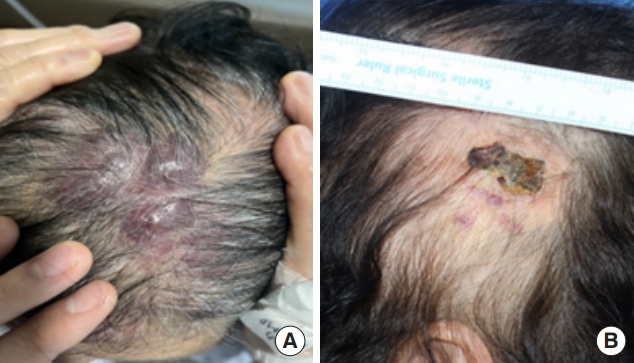
Fig. 2.
An 84-year-old man with recurrent scalp angiosarcoma. (A) Preoperative view. (B) Extensive defect following wide resection surgery. (C) Reconstruction with a latissimus dorsi muscle free flap. (D) Recurrence of angiosarcoma 4 years after the initial reconstruction.

Table 1.
Known risk factors of angiosarcoma
REFERENCES
2. Lahat G, Dhuka AR, Hallevi H, Xiao L, Zou C, Smith KD, et al. Angiosarcoma: clinical and molecular insights. Ann Surg 2010;251:1098-106.

3. Mark RJ, Poen JC, Tran LM, Fu YS, Juillard GF. Angiosarcoma: a report of 67 patients and a review of the literature. Cancer 1996;77:2400-6.


5. Sturm EC, Marasco IS, Katz SC. Multidisciplinary management of angiosarcoma: a review. J Surg Res 2021;257:213-20.


6. Florou V, Wilky BA. Current management of angiosarcoma: recent advances and lessons from the past. Curr Treat Options Oncol 2021;22:61.



7. Coindre JM, Terrier P, Guillou L, Le Doussal V, Collin F, Ranchere D, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the french federation of cancer centers sarcoma group. Cancer 2001;91:1914-26.


8. Rouhani P, Fletcher CD, Devesa SS, Toro JR. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer 2008;113:616-27.


9. Albores-Saavedra J, Schwartz AM, Henson DE, Kostun L, Hart A, Angeles-Albores D, et al. Cutaneous angiosarcoma: analysis of 434 cases from the Surveillance, Epidemiology, and End Results Program, 1973-2007. Ann Diagn Pathol 2011;15:93-7.


10. Brenn T, Fletcher CD. Radiation-associated cutaneous atypical vascular lesions and angiosarcoma: clinicopathologic analysis of 42 cases. Am J Surg Pathol 2005;29:983-96.

11. Yap J, Chuba PJ, Thomas R, Aref A, Lucas D, Severson RK, et al. Sarcoma as a second malignancy after treatment for breast cancer. Int J Radiat Oncol Biol Phys 2002;52:1231-7.


12. Fayette J, Martin E, Piperno-Neumann S, Le Cesne A, Robert C, Bonvalot S, et al. Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 cases. Ann Oncol 2007;18:2030-6.


13. Weiss SW, Goldblum JR. Enzinger and weiss’s soft tissue tumors. 5th ed. Mosby; 2008.
14. Stewart FW, Treves N. Lymphangiosarcoma in postmastectomy lymphedema: a report of six cases in elephantiasis chirurgica. Cancer 1948;1:64-81.


15. Mery CM, George S, Bertagnolli MM, Raut CP. Secondary sarcomas after radiotherapy for breast cancer: sustained risk and poor survival. Cancer 2009;115:4055-63.


16. Manner J, Radlwimmer B, Hohenberger P, Mossinger K, Kuffer S, Sauer C, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol 2010;176:34-9.



17. Huang J, Mackillop WJ. Increased risk of soft tissue sarcoma after radiotherapy in women with breast carcinoma. Cancer 2001;92:172-80.


18. West JG, Weitzel JN, Tao ML, Carpenter M, West JE, Fanning C. BRCA mutations and the risk of angiosarcoma after breast cancer treatment. Clin Breast Cancer 2008;8:533-7.


19. Chaudhary P, Bhadana U, Singh RA, Ahuja A. Primary hepatic angiosarcoma. Eur J Surg Oncol 2015;41:1137-43.


20. Bosetti C, La Vecchia C, Lipworth L, McLaughlin JK. Occupational exposure to vinyl chloride and cancer risk: a review of the epidemiologic literature. Eur J Cancer Prev 2003;12:427-30.


21. Karkouche R, Kerob D, Battistella M, Soufir N, Hadj-Rabia S, Bagot M, et al. Angiosarcoma in patients with xeroderma pigmentosum: less aggressive and not so rare? J Am Acad Dermatol 2013;69:e142-3.


22. Qureshi YA, Strauss DC, Thway K, Fisher C, Thomas JM. Angiosarcoma developing in a non-functioning arteriovenous fistula post-renal transplant. J Surg Oncol 2010;101:520-3.



23. Shustef E, Kazlouskaya V, Prieto VG, Ivan D, Aung PP. Cutaneous angiosarcoma: a current update. J Clin Pathol 2017;70:917-25.


24. Morgan MB, Swann M, Somach S, Eng W, Smoller B. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol 2004;50:867-74.


25. Ohsawa M, Naka N, Tomita Y, Kawamori D, Kanno H, Aozasa K. Use of immunohistochemical procedures in diagnosing angiosarcoma: evaluation of 98 cases. Cancer 1995;75:2867-74.


26. Behjati S, Tarpey PS, Sheldon H, Martincorena I, Van Loo P, Gundem G, et al. Recurrent PTPRB and PLCG1 mutations in angiosarcoma. Nat Genet 2014;46:376-9.




27. Murali R, Chandramohan R, Moller I, Scholz SL, Berger M, Huberman K, et al. Targeted massively parallel sequencing of angiosarcomas reveals frequent activation of the mitogen activated protein kinase pathway. Oncotarget 2015;6:36041-52.



28. Udager AM, Ishikawa MK, Lucas DR, McHugh JB, Patel RM. MYC immunohistochemistry in angiosarcoma and atypical vascular lesions: practical considerations based on a single institutional experience. Pathology 2016;48:697-704.


29. Pawlik TM, Paulino AF, McGinn CJ, Baker LH, Cohen DS, Morris JS, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer 2003;98:1716-26.


30. Abraham JA, Hornicek FJ, Kaufman AM, Harmon DC, Springfield DS, Raskin KA, et al. Treatment and outcome of 82 patients with angiosarcoma. Ann Surg Oncol 2007;14:1953-67.



31. Fury MG, Antonescu CR, Van Zee KJ, Brennan MF, Maki RG. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J 2005;11:241-7.

32. Naka N, Ohsawa M, Tomita Y, Kanno H, Uchida A, Aozasa K. Angiosarcoma in Japan: a review of 99 cases. Cancer 1995;75:989-96.


33. Lahat G, Dhuka AR, Lahat S, Smith KD, Pollock RE, Hunt KK, et al. Outcome of locally recurrent and metastatic angiosarcoma. Ann Surg Oncol 2009;16:2502-9.



34. Mocellin S, Rossi CR, Brandes A, Nitti D. Adult soft tissue sarcomas: conventional therapies and molecularly targeted approaches. Cancer Treat Rev 2006;32:9-27.


35. Grassia KL, Peterman CM, Iacobas I, Margolin JF, Bien E, Padhye B, et al. Clinical case series of pediatric hepatic angiosarcoma. Pediatr Blood Cancer 2017;64:e26627.


36. Guadagnolo BA, Zagars GK, Araujo D, Ravi V, Shellenberger TD, Sturgis EM. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck 2011;33:661-7.



37. Dettenborn T, Wermker K, Schulze HJ, Klein M, Schwipper V, Hallermann C. Prognostic features in angiosarcoma of the head and neck: a retrospective monocenter study. J Craniomaxillofac Surg 2014;42:1623-8.


38. Lindet C, Neuville A, Penel N, Lae M, Michels JJ, Trassard M, et al. Localised angiosarcomas: the identification of prognostic factors and analysis of treatment impact: a retrospective analysis from the french sarcoma group (GSF/GETO). Eur J Cancer 2013;49:369-76.


39. Cassidy RJ, Switchenko JM, Yushak ML, Madden N, Khan MK, Monson DK, et al. The importance of surgery in scalp angiosarcomas. Surg Oncol 2018;27:A3-8.



40. Sinnamon AJ, Neuwirth MG, McMillan MT, Ecker BL, Bartlett EK, Zhang PJ, et al. A prognostic model for resectable soft tissue and cutaneous angiosarcoma. J Surg Oncol 2016;114:557-63.


41. Mullins B, Hackman T. Angiosarcoma of the head and neck. Int Arch Otorhinolaryngol 2015;19:191-5.



42. Deyrup AT, McKenney JK, Tighiouart M, Folpe AL, Weiss SW. Sporadic cutaneous angiosarcomas: a proposal for risk stratification based on 69 cases. Am J Surg Pathol 2008;32:72-7.


43. Vorburger SA, Xing Y, Hunt KK, Lakin GE, Benjamin RS, Feig BW, et al. Angiosarcoma of the breast. Cancer 2005;104:2682-8.


44. Choi JH, Ahn KC, Chang H, Minn KW, Jin US, Kim BJ. Surgical treatment and prognosis of angiosarcoma of the scalp: a retrospective analysis of 14 patients in a single institution. Biomed Res Int 2015;2015:321896.




45. Harati K, Lehnhardt M. The changing paradigm of resection margins in sarcoma resection. Innov Surg Sci 2017;2:165-70.



46. Patel SH, Hayden RE, Hinni ML, Wong WW, Foote RL, Milani S, et al. Angiosarcoma of the scalp and face: the Mayo Clinic experience. JAMA Otolaryngol Head Neck Surg 2015;141:335-40.


47. Shin JY, Roh SG, Lee NH, Yang KM. Predisposing factors for poor prognosis of angiosarcoma of the scalp and face: systematic review and meta-analysis. Head Neck 2017;39:380-6.



48. Sarcoma Meta-analysis Collaboration. Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: metaanalysis of individual data. Lancet 1997;350:1647-54.


49. DeMartelaere SL, Roberts D, Burgess MA, Morrison WH, Pisters PW, Sturgis EM, et al. Neoadjuvant chemotherapy-specific and overall treatment outcomes in patients with cutaneous angiosarcoma of the face with periorbital involvement. Head Neck 2008;30:639-46.


50. Heinhuis KM, IJzerman NS, van der Graaf WT, Kerst JM, Schrage Y, Beijnen JH, et al. Neoadjuvant systemic treatment of primary angiosarcoma. Cancers (Basel) 2020;12:2251.



51. Young RJ, Natukunda A, Litiere S, Woll PJ, Wardelmann E, van der Graaf WT. First-line anthracycline-based chemotherapy for angiosarcoma and other soft tissue sarcoma subtypes: pooled analysis of eleven european organisation for research and treatment of cancer soft tissue and bone sarcoma group trials. Eur J Cancer 2014;50:3178-86.


52. D’Angelo SP, Munhoz RR, Kuk D, Landa J, Hartley EW, Bonafede M, et al. Outcomes of systemic therapy for patients with metastatic angiosarcoma. Oncology 2015;89:205-14.




53. Penel N, Bui BN, Bay JO, Cupissol D, Ray-Coquard I, Piperno-Neumann S, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX study. J Clin Oncol 2008;26:5269-74.


54. Schlemmer M, Reichardt P, Verweij J, Hartmann JT, Judson I, Thyss A, et al. Paclitaxel in patients with advanced angiosarcomas of soft tissue: a retrospective study of the EORTC soft tissue and bone sarcoma group. Eur J Cancer 2008;44:2433-6.


55. Fata F, O’Reilly E, Ilson D, Pfister D, Leffel D, Kelsen DP, et al. Paclitaxel in the treatment of patients with angiosarcoma of the scalp or face. Cancer 1999;86:2034-7.


56. Stacchiotti S, Palassini E, Sanfilippo R, Vincenzi B, Arena MG, Bochicchio AM, et al. Gemcitabine in advanced angiosarcoma: a retrospective case series analysis from the italian rare cancer network. Ann Oncol 2012;23:501-8.


57. Tian Z, Zhang F, Li P, Wang J, Yang J, Zhang P, et al. Albuminbound paclitaxel and gemcitabine combination therapy in soft tissue sarcoma. BMC Cancer 2020;20:698.




58. Fujisawa Y, Fujimura T, Matsushita S, Yamamoto Y, Uchi H, Otsuka A, et al. The efficacy of eribulin mesylate for patients with cutaneous angiosarcoma previously treated with taxane: a multicentre prospective observational study. Br J Dermatol 2020;183:831-9.



59. Ravi V, Wagner M, Chen TW, Loong HH, Mennel RG, Yen CC, et al. A phase II study of oraxol in the treatment of unresectable cutaneous angiosarcoma. J Clin Oncolo 2020;38(15_suppl):11517.

60. Koontz BF, Miles EF, Rubio MA, Madden JF, Fisher SR, Scher RL, et al. Preoperative radiotherapy and bevacizumab for angiosarcoma of the head and neck: two case studies. Head Neck 2008;30:262-6.


61. Agulnik M, Yarber JL, Okuno SH, von Mehren M, Jovanovic BD, Brockstein BE, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol 2013;24:257-63.


62. Dickson MA, D’Adamo DR, Keohan ML, D’Angelo SP, Carvajal RD, Gounder MM, et al. Phase II trial of gemcitabine and docetaxel with bevacizumab in soft tissue sarcoma. Sarcoma 2015;2015:532478.




63. Kollar A, Jones RL, Stacchiotti S, Gelderblom H, Guida M, Grignani G, et al. Pazopanib in advanced vascular sarcomas: an EORTC soft tissue and bone sarcoma group (STBSG) retrospective analysis. Acta Oncol 2017;56:88-92.


64. von Mehren M, Litwin S, Ravi V, Schuetze S, Movva S, Agulnik M, et al. Multicenter phase II trial of pazopanib (P) in patients with angiosarcoma (AS). J Clin Oncol 2019;37(15_suppl):11039.

65. Ravi V, Sanford EM, Wang WL, Ross JS, Ramesh N, Futreal A, et al. Antitumor response of VEGFR2- and VEGFR3-amplified angiosarcoma to pazopanib. J Natl Compr Canc Netw 2016;14:499-502.


66. Mehta CR, Liu L, Theuer C. An adaptive population enrichment phase III trial of TRC105 and pazopanib versus pazopanib alone in patients with advanced angiosarcoma (TAPPAS trial). Ann Oncol 2019;30:103-8.



67. Mir O, Brodowicz T, Italiano A, Wallet J, Blay JY, Bertucci F, et al. Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2016;17:1732-42.


68. Pasquier E, Andre N, Street J, Chougule A, Rekhi B, Ghosh J, et al. Effective management of advanced angiosarcoma by the synergistic combination of propranolol and vinblastine-based metronomic chemotherapy: a bench to bedside study. EBioMedicine 2016;6:87-95.



69. Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 2020;21:1353-65.


70. Painter CA, Jain E, Tomson BN, Dunphy M, Stoddard RE, Thomas BS, et al. The angiosarcoma project: enabling genomic and clinical discoveries in a rare cancer through patient-partnered research. Nat Med 2020;26:181-7.



71. Florou V, Rosenberg AE, Wieder E, Komanduri KV, Kolonias D, Uduman M, et al. Angiosarcoma patients treated with immune checkpoint inhibitors: a case series of seven patients from a single institution. J Immunother Cancer 2019;7:213.




72. Momen S, Fassihi H, Davies HR, Nikolaou C, Degasperi A, Stefanato CM, et al. Dramatic response of metastatic cutaneous angiosarcoma to an immune checkpoint inhibitor in a patient with xeroderma pigmentosum: whole-genome sequencing aids treatment decision in end-stage disease. Cold Spring Harb Mol Case Stud 2019;5:a004408.



73. Hamacher R, Kampfe D, Reuter-Jessen K, Pottgen C, Podleska LE, Farzaliyev F, et al. Dramatic response of a PD-L1-positive advanced angiosarcoma of the scalp to pembrolizumab. JCO Precis Oncol 2018;2:1-7.

74. Huis In ‘t Veld EA, Grunhagen DJ, Verhoef C, Smith HG, van Akkooi ACJ, Jones R, et al. Isolated limb perfusion for locally advanced angiosarcoma in extremities: a multi-centre study. Eur J Cancer 2017;85:114-21.


75. Park YS, Kim JH, Kim KW, Lee IS, Yoon HK, Ko GY, et al. Primary hepatic angiosarcoma: imaging findings and palliative treatment with transcatheter arterial chemoembolization or embolization. Clin Radiol 2009;64:779-85.


- TOOLS
-
METRICS

-
- 0 Crossref
- Scopus
- 1,074 View
- 34 Download
- Related articles in ACFS
-
Current concepts of craniofacial fibrous dysplasia: pathophysiology and treatment2023 April;24(2)
Current concepts of Kimura disease: pathophysiology and evolution of treatment2022 December;23(6)
Current concepts of neurofibromatosis type 1: pathophysiology and treatment2022 February;23(1)






