 |
 |
- Search
| Arch Craniofac Surg > Volume 20(4); 2019 > Article |
|
Abstract
Necrotizing fasciitis (NF) is a rapidly progressive necrosis of the subcutaneous tissue and fascia, caused by bacterial infection. Usually presenting in the extremities, trunk, or perineum, it is uncommon in the craniofacial or cervical area. Cervicofacial NF is a potentially fatal infection, which should be managed with early detection and intervention. Most cases have a primary odontogenic source of infection, especially when the masticator space is involved. We report a case of masticator space NF that developed without odontogenic origin in a 78-year old female who was treated with prompt surgical drainage and intravenous antibiotics.
Necrotizing fasciitis (NF) is an uncommon progressive infection of the fascia and the subcutaneous tissues with high mortality and morbidity rate. The term “necrotizing fasciitis” was first mentioned by Wilson (1952) to emphasize that fascial necrosis was the key feature in the progression of the disease and in the majority of his own cases a beta-hemolytic Staphylococcus was the most common pathogen isolated [1]. Subsequent studies with sophisticated methods of bacteriological investigations have shown the majority of cases to be due to mixed pathogens [2].
NF is well-recognized in various parts of the body like the trunk or the extremities but is less common in the head and neck region [3,4]. NF of the head and neck is most commonly referred to as cervicofacial NF (CNF) or craniocervical NF. CNF is often a polymicrobial infection including both anaerobic and aerobic species. Although incidence has not been readily reported as it is a rare condition, CNF has been reported to reach 2.6% to 5% among all cases of NF [5,6]. The paucity of reports and lower incidence is probably due to the high vascularity of the head and neck region.
When CNF arises, it is typically from odontogenic origin [7]. There are other causes including oropharyngeal infection such as tonsillitis and peritonsillar abscess, insect bites, trauma and postoperative infection [4,8]. In this study, we present a case of a CNF in the masticator space accompanied by mandibular osteomyelitis that developed without evidence of odontogenic origin in an edentulous 78-year old female. She was successfully treated with extensive debridement and intravenous antibiotics.
A 78-year-old female with underlying history of hypertension presented at the emergency department with a right sided facial swelling that had begun 1 week ago, extending from the temple down to the submandibular area. She was under hypertensive medication but did not include any anticoagulant or antiplatelet agents. Erythema and redness of the overlying skin was seen without crepitation or signs of skin necrosis (Fig. 1A). She had sustained a minor contusion on her right cheek 2 weeks ago that had caused minor swelling but resolved within a few days. She was febrile with a white cell count of 18,690/μL and C-reactive protein of 20.69 mg/L. According to the Laboratory Risk Indicator for Necrotizing Fasciitis scoring system, she scored 7 points, which was greater than the cutoff value 6 for high suspicion for NF (Table 1). Her laboratory values showed no evidences of hematological problems or signs of bleeding tendency. Oral examination revealed trismus, limitation in mouth opening, and edentulous gums. She had been edentulous for over 20 years. Dental examinations showed that the roots of her #15 to #17 teeth were retained without any sign of infection or abscess formation. Computed tomography showed an abscess of the right masticator and parapharyngeal spaces with extensive gas formation involving the temporalis, masseter and buccinators muscles. Air densities were found in the right mandible suggesting osteomyelitis. There was no evidence of fracture (Figs. 2, 3).
After immediate initiation of intravenous antibiotics (ceftazidime and clindamycin), the patient underwent surgical drainage and debridement of necrotic tissue through temporal, cheek and intraoral incisions (Fig. 1B). An extensive cavity expanding from the temple to the submandibular area was necrotizing at the level of masticator fascia layer. We removed necrotic fascial tissue, and drained around 50 mL of foul odored green and brown pus. Gauze drains were placed through all incisions to maintain drainage.
Blood cultures showed no growth of bacteria and wound swab cultures showed a heavy growth of group A beta-hemolytic Staphylococcus. Under the recommendation of the infection department, intravenous antibiotics were changed to ceftriaxone and clindamycin. She returned to the operating room for sequential debridement and continued to improve until at 21 days after admission, closure of all incisions was done. She was discharged from hospital at 38 days after admission with normal lab counts with no signs of infection.
Despite the gradual improvement in antibiotic treatment and identification of the pathogens, the outcome of the CNF can be fatal and the mortality rate is still high. In a retrospective review of three tertiary care hospitals in Canada reported a mortality up to 20% in NF patients which was comparable to previous studies [9-12].
The mainstay of treatment is surgical and early diagnosis is of utmost importance to lessen the degree or extent of the intervention. Immediate, surgical debridement and antibiotic treatment is mandatory. Tracheostomy may be necessary in patients with airway compromise. Common early signs of CNF include marked cutaneous erythema, warmth, edema, pain disproportionate to clinical sings and hypesthesia. As CNF progresses, patients may develop tissue crepitus, bullae and skin necrosis with purple-black discoloration secondary to blood vessel thrombosis. Early diagnosis and early surgical debridement are essential for better prognosis. As CNF progresses, systemic signs of shock may appear. Sometimes patients present with a poorly refined area of soft tissue infection with normal skin but more often with blue or brown discoloration. Other skin manifestations may include the presence of petechiae and hemorrhagic bullae.
The most common cause of CNF is odontogenic infection, particularly in those patients with chronic disease or those who are immunosuppressed [3,8,13-16]. Other causes include oropharyngeal infection such as tonsillitis and peritonsillar abscess, insect bites, trauma and postoperative infection [4,8].
Infections in the head and neck area can be subdivided into different spaces based on the anatomy of fascia and interconnected compartments. These include the submandibular, submental, parotid, masticator, prestyloid parapharyngeal, prevertebral and retropharyngeal spaces. The masticator space is a potential space that extends vertically from the skull base down to the ramus of the mandible, between the medial pterygoid and the masseter muscles horizontally. It may be subdivided into the masseteric, temporal, and pterygoid compartments, and is as expected associated with muscles of mastication. This space includes important structures such as temporalis muscle, the ramus of the mandible, divisions of the mandibular nerve (V3) and the internal maxillary artery. The masticator space is located posteriorly to the buccal space, anterior to the parotid space, superiorly to the submandibular space and laterally to the prestyloid parapharyngeal space [17]. Most of infections in this area are odontogenic or origin from an aggressive sinusitis in the maxillary area. The interconnection between the spaces facilitates the rapid spread of infection. Odontogenic infections may initiate from the pulpal and periodontally involved tooth, or the bed of an extracted tooth up to 3 weeks after extraction. Thus, edentulous patients are known to have a very low risk of oral bacterial infection [18]. The patient, in this case, had been edentulous for over 20 years, and we speculate that the preceding contusion that she reported caused post-traumatic accumulation of hemorrhagic fluids, which probably provided a source for rapid bacterial growth and spread throughout the masticator space, eventually reaching the mandible.
Thus, we emphasize that, as in this case, CNF can occur with no apparent odontogenic origin. Even in patients that deny any previous history of dental disease, CNF should be a first line consideration in all patients with suspicious soft tissue infections of the head and neck region and be promptly treated with early surgical debridement and broad-spectrum antibiotics.
Notes
Fig. 1.
Preoperative and postoperative clinical photographs. (A) The patient at presentation. Swelling and redness of the right mandibular angle and temporal areas are seen. (B) The patient during subsequent irrigation. Incisions at the upper and lower aspects of the necrotizing fasciitis cavity are seen. Resolution of swelling is seen.
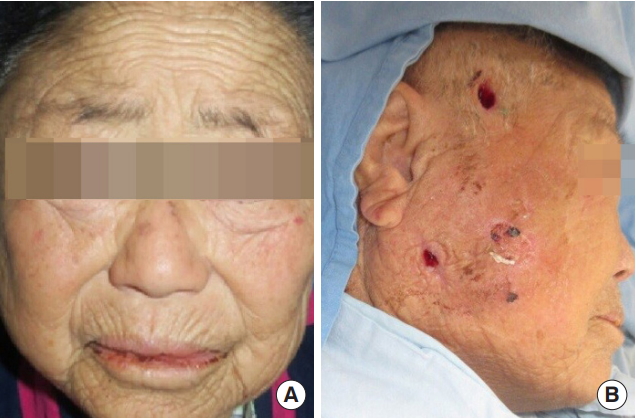
Fig. 2.
Initial contrast-enhanced computed tomography scans. (A) Abscess formation in the right masticator and parapharyngeal spaces with extensive gas formation involving the temporalis, masseter and buccinators muscles. (B) Air densities were found in the right mandible suggesting osteomyelitis.

Fig. 3.
Contrast-enhanced computed tomography scans. (A) Masticator and parapharyngeal spaces (yellow dotted line) in axial view and (B) coronal view.
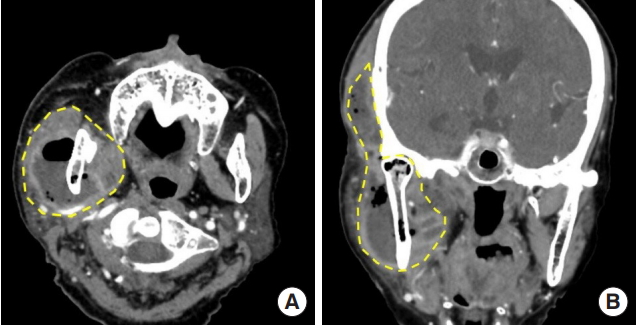
Table 1.
Laboratory Risk Indicator for Necrotizing Fasciitis score
REFERENCES
1. Abdurrazaq TO, Ibikunle AA, Braimah RO. Cervical necrotizing fasciitis: a potentially fatal disease with varied etiology. Ann Med Health Sci Res 2016;6:251-6.



3. Mohammedi I, Ceruse P, Duperret S, Vedrinne J, Bouletreau P. Cervical necrotizing fasciitis: 10 years’ experience at a single institution. Intensive Care Med 1999;25:829-34.



4. Oguz H, Yilmaz MS. Diagnosis and management of necrotizing fasciitis of the head and neck. Curr Infect Dis Rep 2012;14:161-5.



5. Hohlweg-Majert B, Weyer N, Metzger MC, Schon R. Cervicofacial necrotizing fasciitis. Diabetes Res Clin Pract 2006;72:206-8.


6. Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO. Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J Bone Joint Surg Am 2003;85:1454-60.


7. Juncar M, Bran S, Juncar RI, Baciut MF, Baciut G, Onisor-Gligor F. Odontogenic cervical necrotizing fasciitis, etiological aspects. Niger J Clin Pract 2016;19:391-6.


8. Panda NK, Simhadri S, Sridhara SR. Cervicofacial necrotizing fasciitis: can we expect a favourable outcome? J Laryngol Otol 2004;118:771-7.


9. Golger A, Ching S, Goldsmith CH, Pennie RA, Bain JR. Mortality in patients with necrotizing fasciitis. Plast Reconstr Surg 2007;119:1803-7.


10. Faucher LD, Morris SE, Edelman LS, Saffle JR. Burn center management of necrotizing soft-tissue surgical infections in unburned patients. Am J Surg 2001;182:563-9.


11. McHenry CR, Piotrowski JJ, Petrinic D, Malangoni MA. Determinants of mortality for necrotizing soft-tissue infections. Ann Surg 1995;221:558-63.



12. Childers BJ, Potyondy LD, Nachreiner R, Rogers FR, Childers ER, Oberg KC, et al. Necrotizing fasciitis: a fourteen-year retrospective study of 163 consecutive patients. Am Surg 2002;68:109-16.



13. Thakur JS, Verma N, Thakur A, Sharma DR, Mohindroo NK. Necrotizing cervical fasciitis: prognosis based on a new grading system. Ear Nose Throat J 2013;92:149-52.


14. Mathieu D, Neviere R, Teillon C, Chagnon JL, Lebleu N, Wattel F. Cervical necrotizing fasciitis: clinical manifestations and management. Clin Infect Dis 1995;21:51-6.



15. Tung-Yiu W, Jehn-Shyun H, Ching-Hung C, Hung-An C. Cervical necrotizing fasciitis of odontogenic origin: a report of 11 cases. J Oral Maxillofac Surg 2000;58:1347-52.


16. Bakshi J, Virk RS, Jain A, Verma M. Cervical necrotizing fasciitis: our experience with 11 cases and our technique for surgical debridement. Ear Nose Throat J 2010;89:84-6.


- TOOLS
-
METRICS

-
- 7 Crossref
- Scopus
- 4,703 View
- 109 Download
- Related articles in ACFS






