Does Low-Dose Heparin Have a Significant Role in Free Flap Surgery?
Article information
Abstract
Background
It is controversial issue that heparin decreases thrombosis for microsurgical anastomosis, and its effective role is under discussion. This study is for proving whether low-dose heparin is preventing thrombosis in free flap reconstruction.
Methods
Through chart reviews of 134 patients, using low-dose heparin for free tissue transfer from 2011 to 2016, retrospective analysis was performed. 33 patients received low-dose heparin therapy after surgery. And 101 patients received no-heparin therapy. Complications included flap necrosis, hematoma formation, dehiscence and infection.
Results
In no-heparin therapy group, comparing the flap necrosis revealed 16 cases (15.84%). And, flap necrosis was 6 cases (18.18%) in low-dose heparin therapy group. The statistical analysis of flap necrosis rate showed no significant difference (p=0.75). The results showed that there was no significant difference of flap necrosis rate between two groups.
Conclusion
In this study, patients in the low-dose heparin group had no significantly lower rates of flap failure compared with no-heparin group. This suggests that low-dose heparin may not prevent thrombosis and subsequent flap failure to a significant extent.
INTRODUCTION
Currently, free flap is considered to be a common and reliable surgical technique for covering large defects [12]. However, about 1%–4% of patients who underwent free tissue transfer had suffered flap failure due to microvascular thrombosis, anastomotic failure, mechanical obstruction, tension and compression [345]; therefore, improvement is still necessary. Especially, in flap failure with thrombosis, arterial thrombus generally develops during the first 24 hours and venous thrombus forms in the second postoperative day [67].
It is common for prophylactic antithrombotic agents to be used to prevent microvascular thrombosis in the free flap [89]. Many surgeons considered several methods of antithrombotic pathways clinically: Antiplatelet method, anticoagulant method, and blood viscosity reduction method and so on [1011121314]. However, according to the recent study, they suggested that these antithrombotics could not reduce the risk of total flap loss or thrombosis, and they could increase the risk of hematoma [15].
In this study, we clinically researched patients who underwent free tissue transfer surgery and analyzed the results based on the perioperative administration of low-dose heparin for 5 or 7 days, involving the period in which thrombosis typically occurred after free flap reconstruction [6]. Moreover, we evaluated whether the use of low-dose heparin decreases microvascular thrombosis.
METHODS
This retrospective study reviewed 134 cases between 2011 and 2016. During this period, two departments, Plastic and Reconstructive Surgery and Orthopedic Surgery, performed 134 free tissue transfers in a single medical center.
According to perioperative managements, we divided these patients into two groups: one group included 33 patients who received low-dose heparin therapy that was bolus of 2,500 injection units (IU) in the operation room and postoperative infusion at the rate of 200 IU/hour for 7 days [10], and the other group included 101 patients who did not receive heparin therapy. Patient factors, like severity of the disease, were not considered in these two groups. And whether we apply to use low-dose heparin or not was decided by the period of the surgery. We did not use low-dose heparin before 2015 and from January 2015, we used the low-dose heparin.
Patient demographics, age, sex, and body mass index were evaluated. Risk factors, such as diabetes mellitus, hypertension, cardiovascular accident, cerebrovascular disease, and smoking, were considered. Regarding the complications, we deducted a correlation between the two groups with respect to the results of flap necrosis, hematoma formation, dehiscence, and infection. The extent of flap necrosis included all partial and total necrosis. In these two groups, patients with risk factors were further analyzed for the flap necrosis rate. Statistical analysis of the results was performed using chi-square test. A p-value of less than 0.05 was considered statistically significant.
RESULTS
Demographic analysis is shown in Table 1. In the no-heparin group, the mean age was 55 years old and 52 years old in the low-dose heparin therapy group. The ratio of male to female was 3.21 in the no-heparin group and 2.67 in the low-dose heparin group. In comparison to the ratio of each risk factor between two groups, there was no significant difference on the risk of vascular insufficiency in two groups.
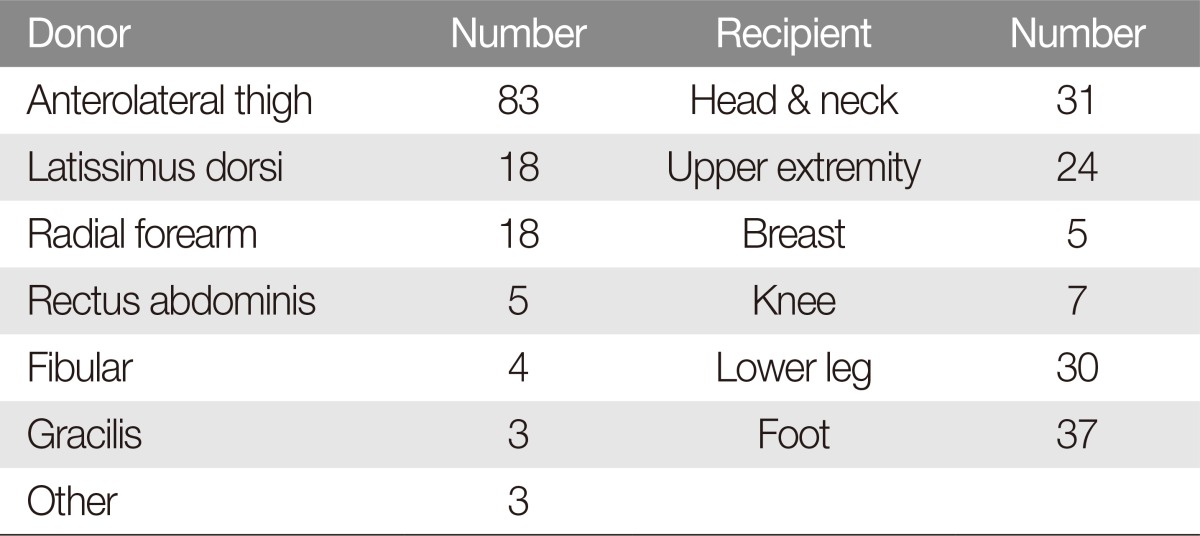
In this study, 31 cases (23.13%) were received on head and neck, 24 (17.91%) were on upper extremities, 30 (22.39%) were on lower legs, 37 (27.61%) were on feet (Table 2). In the cases of head and neck, flap necrosis revealed 6 cases (19.4%), and 16 cases (21.6%) was flap necrosis in low extremity included in knee, lower legs and feet.
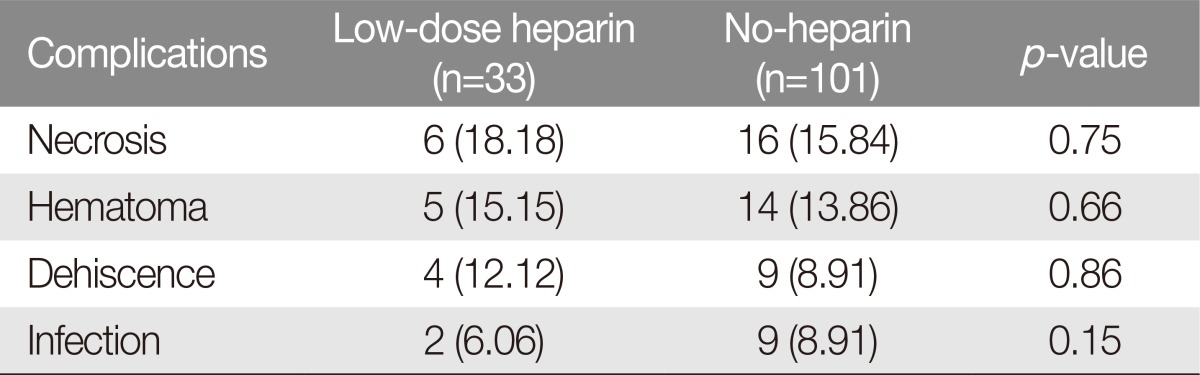
The overall flap necrosis rate was 6 cases (18.18%) in the low-dose heparin group and 16 cases (15.84%) in the no-heparin group. The flap necrosis rate showed no significant difference (p=0.75; odds ratio, 1.18; 95% confidence interval [CI], 0.42 to 3.32) (Table 3). There was no significant difference regarding the complications of hematoma formation (p=0.66; odds ratio, 0.80; 95% CI, 0.29 to 2.18), dehiscence, and infection (p=0.86; p=0.15) (Table 3). There were 5 cases (15.15%) in the low-dose heparin group and 14 cases with hematoma (13.86%) in the no-heparin group. The number of dehiscence cases was 9 (8.91%) and 4 (12.12%), respectively; the number of infection cases was 9 (8.91%) and 2 (6.06%), respectively.
Risk factors of flap necrosis in patients were analyzed (Table 4). There was no significant difference (p=0.98; p=0.97; p=0.92) of the flap necrosis rate regarding diabetes mellitus, hypertension, smoking, cardiovascular accident, and cerebrovascular disease between the two groups. Low-dose heparin did not have a positive effect on the increase of flap survival rate in high-risk patients compared with the controls.
DISCUSSION
After suggesting that antithrombotic agents could increase the patency of vessels in the free flap [16], microsurgeons have applied these agents to improve flap necrosis. Although there have been various studies to date evaluating whether antithrombotics can prevent flap failure in microvascular surgery [1718192021222324], little clinical evidence has been demonstrated. Nevertheless, about 96% of microsurgeons actually apply antithrombotic agents for free flaps [11].
Insufficient clinical evidence may be due to various antithrombotic agents and protocols being applied by various medical center and microsurgeons [1718192021222324]. Among them, we emphasized what we have applied in our medical center; low-dose heparin (bolus of 2,500 IU in the operation room and postoperative infusion at the rate of 200 IU/hour for 7 days), which was known to decrease flap necrosis rate and have low incidence of hematoma formation [10].
The main role of heparin is to encourage binding of antithrombin III to thrombin, and to inactivate other coagulation factors, such as Xa [25]. Due to the inactivation, fibrinogen cannot be changed to fibrin, and as a result, it leads to a decrease in the formation of blood clot or thrombus [10].
In this study, we investigated whether low-dose heparin is related to the improvement of flap necrosis. There was no significant difference in the flap necrosis rates between low-dose heparin group and no-heparin group. This suggests that heparin may not be the main responsible factor for the prevention of flap necrosis. It is because flap necrosis was not decided by only one factor such as thrombosis. There are various factors related to flap necrosis such as kinks, twists, and tension of the pedicle or flap [26].
In a comparison between the two groups, the low-dose heparin group did not show a significantly increased rate of hematoma formation. This suggests that low-dose heparin has no significant effect on the formation of hematoma. This is confirmed by the following. The hematoma rate of low-dose heparin group was the same as that of no-heparin group, and in Kroll et al.'s [10] study, the hematoma rate of low-dose heparin group was significantly lower than that of high-dose heparin group.
Our study has several strengths compared with other studies. In many studies, the most common recipient site for the free flap was the head and neck [1213202224], followed by the breast or lower extremity [182224]. We applied the free flap not only to the head and neck, but also to the lower leg, foot, and hand. Moreover, by using low-dose heparin, we found a lower rate of complications, such as hematoma formation.
However, there are some limitations to consider in this present study. First, the chart review was performed retrospectively. However, the data related to flap failure and hematoma formation in the free flap were collected prospectively; therefore, we increased the probability of data. Second, the use of low-dose heparin was dependent on the preference of each surgeon. Although there were various methods, such as intraoperative bolus only, postoperative high-dose heparin infusion etc., the most effective method with low-dose heparin was used during the perioperative period [10]. Third, prostaglandin-E1 was also used in all cases. Therefore, there was no control group in which the prostaglandin-E1 was not used. Fourth, there were a few confounding factors between the two groups. Although the difference was not statistically significant, the no-heparin group had more diabetes patients and hypertension patients than the other group.
Our study suggests that low-dose heparin may not play the main role in influencing the outcome of free flaps. Patients not receiving heparin showed a comparable free flap survival rate. Postoperative low-dose heparin therapy may not be a useful therapeutic option in patients undergoing microvascular reconstruction.
Notes
No potential conflict of interest relevant to this article was reported.