Temporal augmentation with calvarial onlay graft during pterional craniotomy for prevention of temporal hollowing
Article information
Abstract
Background
Atrophy of muscle and fat often contributes to temporal hollowing after pterional craniotomy. However, the main cause is from the bony defect. Several methods to prevent temporal hollowing have been introduced, all with specific limitations. Autologous bone grafts are most ideal for cranial defect reconstruction. The authors investigated the effectiveness of bony defect coverage and temporal augmentation using pterional craniotomy bone flap.
Methods
This study was conducted in 100 patients who underwent brain tumor excision through pterional approach from 2015 to 2016. Group 1 underwent pterional craniotomy with temporal augmentation and group 2 without temporal augmentation. In group 1, after splitting the calvarial bone at the diploic space, the inner table was used for covering the bone defect and as an onlay graft for temporal augmentation. The outcome is evaluated by computed tomography at 1-year follow-up.
Results
The mean operative time for temporal augmentation was 45 minutes. The mean follow-up was 12 months. The ratio of temporal thickness of operated side to non-operated side was 0.99 in group 1 and 0.44 in group 2, which was statistically different. The mean visual analogue scale score was 1.77 in group 1 and 6.85 in group 2.
Conclusion
This study demonstrated a surgical technique using autologous bone graft for successfully preventing the temporal hollowing and improved patient satisfaction.
INTRODUCTION
Temporal hollowing is a frequent complication after pterional craniotomy. Temporal hollowing often occurs within 6 months post-operatively and can be a significant aesthetic concern. It can be associated with pain due to the indentation of the temporalis muscle and adhesions to the dura [1] and may result in a delayed return to daily activities and adverse rehabilitation outcomes. With improvements in neurosurgical outcomes, postoperative cosmesis has received an increased emphasis in cranial operations.
There are many culprits of temporal hollowing such as atrophy of temporalis muscle or temporal fat pad from devascularization. However, the most fundamental cause appears to be the bony defect of the temporal region from craniotomy. Several methods to prevent or treat temporal hollowing following pterional craniotomy have been suggested, but all have specific limitations [2,3]. Many allograft substances have been utilized in cranial reconstruction, including methyl methacrylate, titanium mesh, porous polyethylene, and hydroxyapatite cement [4,5].
The senior author had previously presented a primary temporal reconstruction technique using one autologous bone graft for coverage of temporal defect and temporal area [6]. This technique successfully prevented temporal hollowing, but the bony plate positioning was limited due to its large size. And, the other limitation of the study was a small sample size. The goal of this study was to complement these limitations by introducing a method of covering the temporal bony defect by using an autologous calvarial bone graft and, at the same time, augmenting the temporal fossa with a separate autologous bone graft. In addition, we present the outcomes between patients who underwent this procedure compared to those who did not.
METHODS
Patients who underwent pterional craniotomy at the time of intracranial tumor excision at the Bundang Jesaeng General Hospital from January 1, 2015 to December 31, 2016 were included in this retrospective study. Patients who had recurrence of the brain tumor, those who had preoperational temporal hollowing, or patients with less than 1-year follow-up were excluded from the study. Patients were divided into two groups: (1) group 1 underwent pterional craniotomy with temporal augmentation and (2) group 2 underwent pterional craniotomy without temporal augmentation. All patients in group 1 consented for the temporal augmentation.
The collected demographic information included patient age, sex, body mass index, and past medical/surgical histories. Intraoperative details, including the size of bony defect, the size of craniotomy bone segment, the size and thickness of calvarial onlay graft, and tumor pathology were documented. Radiologic studies were reviewed, and preoperative and postoperative photographs were analyzed for the subjective assessment of temporal hollowing. The patients visited our clinic at 3 months, 6 months, and 12 months after surgery. All patients included in the study had clinical photographs and computed tomography (CT) studies at 12 months postoperatively.
The 12-month postoperative CT imaging was used to evaluate the degree of temporal hollowing. To quantify the severity of temporal hollowing, we measured the distance of depression at the level of temporal fossa from the coronal CT image. This distance, “temporal thickness,” was defined as the shortest distance between the skin surface and a line drawn from the lateral orbital rim tangentially to the temporal bone (Fig. 1). The blue line is a horizontal line drawn from the deepest point of the temporal fossa. The yellow line is a line from lateral orbital rim to the tangential point on the temporal bone. The green line originates from the intersection between the skin surface and the blue, and drawn parallel to the yellow line. The red line (temporal thickness) is the shortest distance between the yellow line and the green line. This measurement was made bilaterally and the difference between the temporal thickness of operated and non-operated sides was measured in all patients (Fig. 1). All patients included in this study had no temporal hollowing prior to the surgery, therefore the difference between the temporal thickness of operated side and nonoperated side represented temporal hollowing. We set the grade of temporal hollowing from 0 to 3: the difference less than 10% between the temporal thickness of operated side and non-operated side was designated to grade 0 (no hollowing); the difference of 10%–25% was designated to grade 1 (mild hollowing); the difference of 25%–50% was designated to grade 2 (moderate hollowing). Grade 3 (severe hollowing) was used if the difference was more than 50% (Table 1). Patient satisfaction was assessed by the subjective assessment of temporal hollowing, which was evaluated on visual analogue scale (VAS) from 0 (no deformity) to 10 (severe temporal hollowing) at 12 months postoperatively.

Measurement of temporal thickness via computed tomography coronal image. Temporal thickness is defined as the shortest distance between the skin surface and a line drawn from the lateral orbital rim tangentially to the temporal bone. The blue line is a horizontal line drawn from the deepest point of the temporal fossa. The yellow line is a line from lateral orbital rim to the tangential point on the temporal bone. The green line originates from the intersection between the skin surface and the blue line, and runs parallel to the yellow line. The red line (temporal thickness) was a shortest distance from the yellow line and the green line. (A) A 74-year-old female in group 1 underwent pterional craniotomy with temporal augmentation on the right side. Temporal thickness was 12.08 mm on operated side and 12.49 mm on non-operated side 12-month after surgery. (B) A 63-year-old female in group 2 underwent pterional craniotomy on the right side without temporal augmentation. Temporal thickness was 3.40 mm on operated side and 13.19 mm on non-operated side 12-months after surgery.
Surgical technique
The scalp incision was designed and the dissection was extended deep until the superficial temporal fascia was encountered. The superficial fascia was then incised and reflected anteriorly along with the frontal periosteum. This maneuver exposed the deep temporal fascia and the temporalis muscle, which were divided with a 1.5 cm myofascial cuff retained along the superior temporal line. With the soft tissue layers dissected and the lateral portion of the cranium exposed, the neurosurgeons performed pterional craniotomy. The size of the temporal bone defect was measured and the bone flap was held in saline-soaked gauze.
The cranial bone flap was usually broad and included the temporal and parietal bones. The parietal segment of the bone flap, which was relatively thicker than the temporal bone, was used for the coverage of the temporal defect and for temporal augmentation. The parietal segment of the bone flap was marked to cover the temporal bony defect, then cut using a reciprocating saw. The inner table of the parietal bone segment was separated from the outer table using an oscillating saw. The resulting outer table was rigidly fixated back in the original position with microplates and screws. The inner table of the parietal segment was used as source material for coverage of bony defect and the onlay graft; it was designed, marked, and then cut further using a reciprocating saw. The portion of the inner table was fixed to the temporal bony defect site with microplates and 4 mm screws, and the remaining inner table (used as the onlay graft as temporal augmentation) was rigidly fixated with 7 mm screws. Sharp edges were smoothed using a cutting burr. The temporalis muscle was reattached to the myofascial cuff at the superior temporal line using 3 0 Vicryl sutures. The scalp was closed primarily in layers (Fig. 2).

Surgical technique of temporal augmentation with calvarial onlay graft. A 48-year-old male with glioma on right temporal lobe underwent pterional craniotomy with temporal augmentation with calvarial onlay graft. (A)The cranial bone flap was separated. (B) Parietal segment of the cranial bone flap was marked and divided. The parietal segment was then split at the diploic space. The outer table was fixed it its original position. (C) The inner table was further divided and used as the flap for defect coverage (yellow arrow) and the onlay graft for temporal augmentation (blue arrow). (D) The reconstructed cranial bone flap was placed back on the patient. a, anterior; c, cephalic; p, posterior; c’, caudal.
Statistical analysis
All statistical analyses were performed using IBM SPSS ver. 24.0 (IBM Corp., Armonk, NY, USA). Continuous data between group 1 and group 2 were compared using two-sample t-tests or Mann-Whitney tests depending on the assumption of normality. Categorical variables were analyzed using chi-square tests. A p-value <0.05 was considered to be a significant difference.
RESULTS
One hundred eighteen patients underwent pterional craniotomy for neurosurgical tumor excision. Eighteen patients were excluded due to recurrence of the brain tumor (n=5) and insufficient follow-up period (n=13). Total 100 patients (38 males and 62 females) were included in this study. Forty-one patients underwent pterional craniotomy with temporal augmentation and 59 patients underwent pterional craniotomy without temporal augmentation. The mean age of the patients was 45.9±13.64 years and 49±13.2 years for group 1 and group 2, respectively. The male-to-female ratio was 1:1.56 and 1:1.68 for group 1 and group 2, respectively (Table 2). Meningioma was the most common tumor in both groups: 39% in group 1 and 46% in group 2. Other tumors included glioma, schwannoma, craniopharyngioma, and astrocytoma. The patients’ mean size of total craniotomy bone segment in group 1 was 102.7±23.4 cm2; the mean size of bone flap for bony defect coverage was 10.28±7.15 cm2; the mean size of calvarial onlay graft was 7.49±1.58 cm2 with the mean thickness of 2.33±0.47 mm (Table 3). The mean operative time of 45 minutes for both groups. Postoperatively soft tissue swelling remained on the operated area at 3 months. The soft tissue swelling resolved by 6 months, and there was no significant clinical difference between at 6 months and 12 months.
The mean size of onlay graft in group 1 was 7.49±1.58 cm2. The mean postoperative temporal thickness of operated side was 13.75±3.05 mm and the thickness of non-operated side was 13.82±2.99 mm. In group 2, the mean postoperative temporal thickness of operated side was 5.80±3.48 mm and the thickness of non-operated side was 13.04±1.97 mm. The ratio of the thickness of operated side to non-operated side was 0.99±0.29 in group 1 and 0.44±0.25 in group 2. The temporal thickness of operated side showed a statistically significant difference between group 1 and group 2 (p <0.001). However, the temporal thickness of non-operated side showed no statistically significant difference between group 1 and group 2 (p =0.195). Since all patients had no temporal hollowing prior to the surgery, the ratio between the temporal thickness of operated side and non-operated side could represent the temporal hollowing. Postoperatively, this ratio showed statistically significant difference between group 1 and group 2 (p <0.001). The mean VAS score was 1.77±1.26 in group 1 and 6.85±2.24 in group 2. Group 1 was significantly lower VAS score than group 2 (p <0.001) (Table 4, Fig. 3).
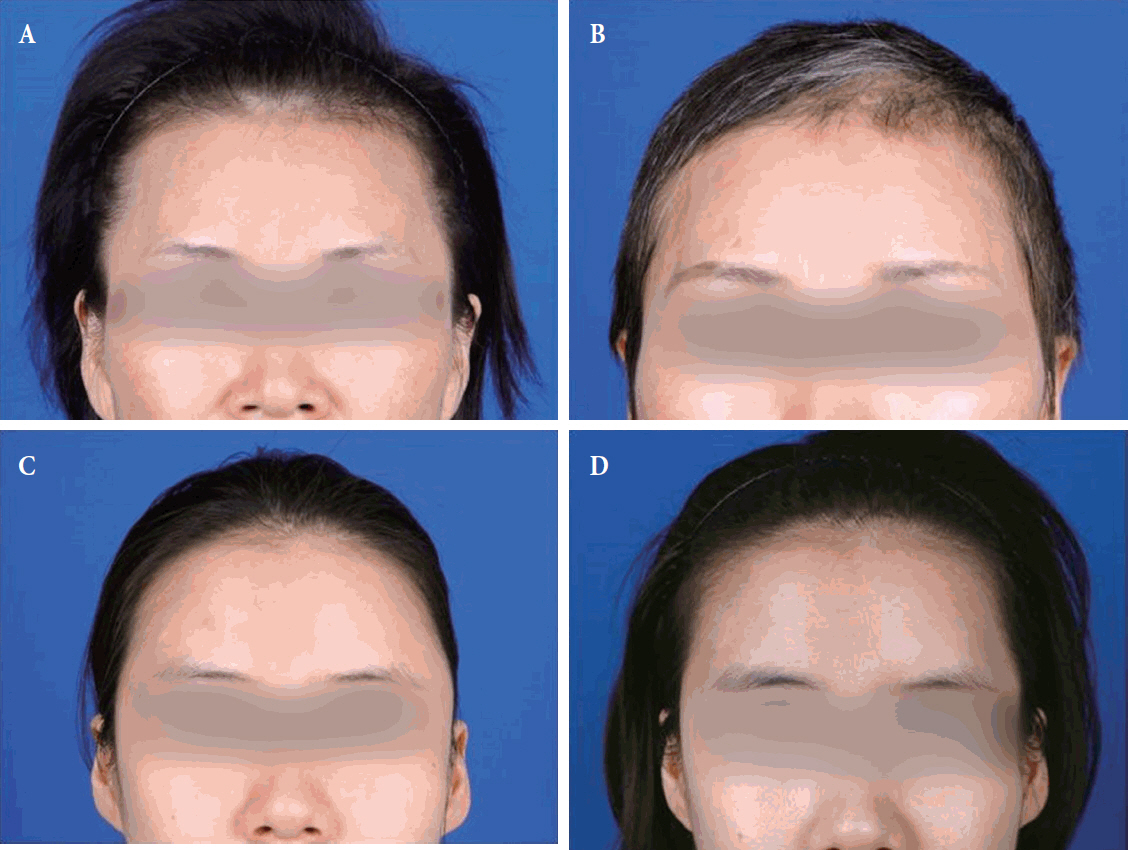
Preoperative and postoperative gross photographs. (A) Preoperative photograph of a 52-year-old female with meningioma on left frontal convexity who underwent pterional craniotomy with temporal augmentation. There was no temporal hollowing before surgery. (B) Postoperative photograph at 12 months of the patient without temporal hollowing. She was very satisfied with the results of the temporal augmentation. (C) Preoperative photograph of a 31-year-old female with meningioma who underwent pterional craniotomy without temporal augmentation. There was no temporal hollowing before surgery. (D) Temporal hollowing was seen at postoperative 12 months.
In group 1, there were 38 patients (93%) with grade 0. Two patients (5%) were grade 1, one patient (2%) was grade 2, and there was no patient with grade 3. In group 2, three patients (5%) were grade 0. Five patients (8%) were grade 1, seven patients (12%) were grade 2, and 44 patients (75%) were grade 3 (Table 5). The patients with mild temporal hollowing (grade 1) felt no discomfort in their daily lives. But the patients with moderate to severe temporal hollowing experienced discomfort and interfered with their social life. Patients in group 1, although mild hollowing occurred in two patients, did not complain of particular discomfort. Moderate hollowing occurred in one patient in group 1, and was successfully treated with local fat grafting. In group 2, moderate to severe hollowing occurred in 51 patients (87%). Marginal skin necrosis occurred in one patient (2%) in group 1 and five patients (8%) in group 2. All resolved with local wound revision. There were two cases of temporal bulging in group 1. One patient had prolapse of fixed temporalis muscle which was corrected by bihalving and refixation of the temporalis muscle. The second patient had hypertrophy of temporal fat pad, which was corrected by partial excision of the excessive fat tissue. No patients had infections or seroma accumulations (Table 6).
DISCUSSION
Pterional craniotomy is the most commonly used neurosurgical approach. Pterional approach is required in many cases, such as excision of brain tumor, repair of cerebral aneurysm and arteriovenous malformation, and access for controlling cerebral hemorrhage. Temporal hollowing is well-known complication after pterional craniotomy. The incidence of hollowing after temporal craniotomy is as high as 87.5%–100% [7-9]. Temporal hollowing appears as a complex result of a variety of causes. It can be caused by the atrophy of temporalis muscle and temporal fat pad. The atrophy may result from number of physiological insults, including direct damage to muscle fibers and fat tissue, vessel damage, and inappropriate tension, malposition or unreliably reattached muscle or fat pad. The extent of atrophy may be directly related to longer operative time by the neurosurgeon, as more atrophy can be caused by the interruption of the neurovascular supply [10-13]. However, the most important underlying cause of the temporal hollowing appears to be the bony defect of the pterional and temporal regions during craniotomy. The presence of the bony defect allows the negative intracranial pressure to “suck in” the temporalis muscle which worsens the temporal hollowing.
The therapeutic approach to temporal hollowing has evolved over the years. For several decades, the patient was instructed to camouflage the concavity by either hairstyling or wearing a hat. These results were often times insufficient, especially for the bald male or during exercise, swimming, and other physical activities [2]. More recently, various methods have been used to treat or prevent temporal hollowing after craniotomy. As a secondary reconstruction at postoperative 6 months, many surgeons often placed implant in the temporal area after temporal hollowing has already occurred. This method has the benefit of correctly compensating for the extent of the temporal hollowing; however, a second operation is required with additional cost. Less invasive treatments such as hyaluronic fillers are available but multiple treatments are usually required and should be considered for patients with limited volume loss. Furthermore, the contour irregularities or granulomas may form which require surgical correction. Autologous fat transfer is an effective alternative with low donor site morbidity for low temporal volume loss. However, one should mindful of contour irregularities and risk of cerebral infarction from intravascular injection of fat, as well as the need for multiple injections due to unpredictable survival of injected fat. The secondary correction requires for the temporal hollowing to manifest fully, which can take up to 6 months [14]. Meanwhile, patients may experience discomfort aesthetically and can interfere with social life.
There are various surgical techniques of augmenting the temporal region at the time of craniotomy to minimize the risk of temporal hollowing. Temporal cranioplasty has been performed using a range of materials, such as acrylics, porous polyethylene, bone cement, titanium, muscle flaps, and prosthetic dermis [15]. These methods are limited by the risk of injuring the surrounding tissue, prolonged preparation phase, the possibility of reabsorption, infection and high cost.
As described above, various methods have been attempted for treatment or prevention of temporal hollowing, but there has been no method of reconstructing the primary temporal bony defect. The most important underlying cause of temporal hollowing is the bony defect of the pterional and temporal regions due to the resection of the sphenoid ridge and temporal squama for adequate exposure. The coverage and augmentation of such bony defects are important in preventing craniofacial deformities and postoperative hollowness. Autologous bone is the most ideal alternative material to date. There is low incidence of infection associated with the use of autologous bone because it possesses the ability to become fully incorporated and revascularized [16]. So far, morbidity of the donor site and increased operative time have curtailed many surgeons from using autologous bone as secondary reconstructive material. Our manuscript demonstrates that temporal augmentation with calvarial onlay graft at the time of tumor excision was not associated with increased morbidity and with similar operative times. Unlike the previous method using one big bone flap, we used two separate bone segments (which was divided from the large bone flap) to cover bony defect and to augment the temporal area. In addition, the current study included 100 patients to improve the reliability of the study [6].
The temporal thickness ratios were significantly different between group 1 and group 2. The authors assigned the grade of the temporal hollowing based on this ratio: the patients in grade 0 and 1 had no discomfort whereas the patients in grade 2 and 3 complained of discomfort. Majority of group 1 patients (93%) had no temporal hollowing (grade 0) and only 2% experienced discomfort (grade >2). In group 2, majority of patients experienced temporal hollowing (95%, grade >1) and discomfort (87%, grade >2). Our data demonstrates that majority of the patients who undergo pterional craniotomy without temporal augmentation will likely result in temporal hollowing. More importantly, our technique can effectively prevent temporal hollowing with bony defect coverage and temporal augmentation using craniotomy bone flap. Furthermore, the VAS score in group 1 (1.77±1.26) reflect high patient satisfaction.
The most common brain tumor in this study was meningioma. Meningioma is common benign tumor in young women which is reflected in our patient population (61% in group 1, 63% in group 2). For this reason, temporal augmentation may be of more aesthetic concern. In our institution, temporal augmentation is performed as part of a single stage neurosurgical operation, thereby decreasing the need for a separate reconstructive operation. The overall rate of complications was low and group 1 patients who had temporal augmentation had less skin necrosis compared to group 2 patients. Two patients who experienced temporal bulging were also corrected with revision surgery and were all satisfied with the results. There were no major complications from the single-stage temporal augmentation. Out data indicate that temporal augmentation with calvarial onlay graft provides high level of satisfaction to the patients.
There were limitations in this study. The temporal area augmented by the onlay graft was temporal fossa lateral to the lateral orbital rim, anterior to hairline and superior to zygomatic arch. The size of the onlay graft was determined by considering the thickness of the contralateral temporal bone and may have measurement error. Therefore, the empirical decision from well-experienced operator was important. In addition, there are potential inaccuracies with manual measurement of the temporal defect. We are currently studying the use of three-dimensional printing technology to accurately measure the contralateral calvarium and aid in achieving symmetry. One of the patients with temporal bulging had fat pad hypertrophy and was corrected after surgery, but the exact cause was not identified. Further in-depth research into the occurrence and cause of hypertrophy, not atrophy, would help to reduce these complications.
Notes
No potential conflict of interest relevant to this article was reported.
Notes
PATIENT CONSENT
The patients provided written informed consent for the publication and the use of their images.