|
 |
- Search
| Arch Craniofac Surg > Volume 20(2); 2019 > Article |
|
Abstract
Merkel cell carcinoma is a rare cutaneous carcinoma, featured by an aggressive clinical course and a mortality rate of 28% at 2 years. A 71-year-old female was affected by a 4.1-cm-wide locally advanced Merkel cell carcinoma of the upper eyelid, previously misdiagnosed as chalazion, with involvement of the extraocular muscles. Although the tumor showed a macroscopic spontaneous regression in size after the incisional biopsy, the mass was treated with neoadjuvant chemotherapy and surgical excision. Good functional and aesthetic result with preservation of the eyeball and absence of tumor recurrence were achieved at 3-year follow-up. In our experience, the combination of the inflammatory cascade due to the incisional biopsy and neoadjuvant chemotherapy led to the regression of a locally advanced large Merkel cell carcinoma of the eyelid.
Merkel cell carcinoma (MCC) is a rare and aggressive cutaneous carcinoma, which originates from the abnormal proliferation of Merkel cells, usually found in the basal layer of the epidermis. This tumor was first described in 1972 by Toker [1]. Recently, a polyomavirus was found to be integrated into the genome of MCC cells and has been postulated to play a role in the pathogenesis and progression of this tumor [2]. In general, the average age at the time of diagnosis of MCC ranges from 66 to 73 years, and about 75% of patients are older than 65 years old [3]. MCC are exceedingly rare in the black population [4].
The distribution of MCC between males and females is reported to be higher in males (61%) than in females [4]. However, the distribution of the MCC of the eyelid seems inverted: higher in females than in males (31%) [5]. According to the review of the National Cancer Database, at the time of presentation, 66% of patients have local disease, 27% have regional lymph node metastases, and 7% have distant metastatic disease [6]. Distant nonregional lymph nodes are the most common sites of metastatic spread, followed by liver, lung, bone, brain, and any other organ [7]. Our aim is to report the successful use of neoadjuvant chemotherapy in a large locally advanced MCC of the eyelid, probably regressed thanks to the combination of the inflammatory reaction in response to incisional biopsy and neoadjuvant chemotherapy.
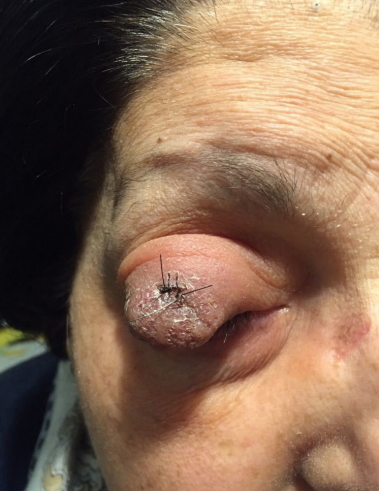
Here we present a case of a 71-year-old female who presented to our department complaining of a mass over the right upper eyelid (Fig. 1). Family history and past medical history were not significant for cancer. She reported that she had first observed the lesion 4 months before we first saw her. Initially her ophthalmologist made a diagnosis of chalazion, hence he prescribed her antibiotic therapy, but without any relief.
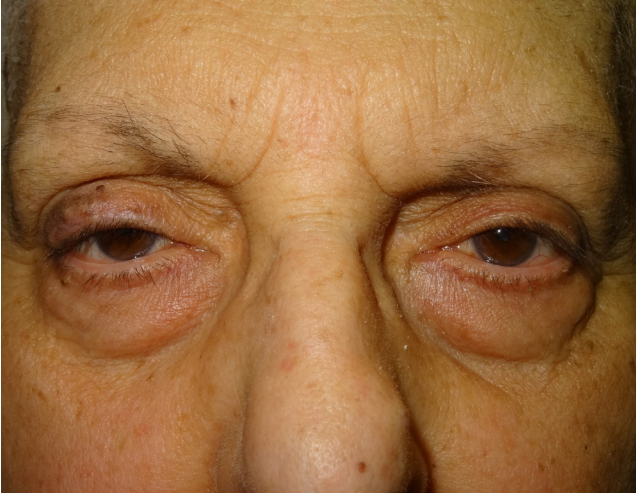
Physical examination revealed a painless voluminous violaceous bulging mass, with nodular irregularities over the surface, which caused mechanical ptosis. According to the clinical suspect of neoplasm, we decided to perform an incisional biopsy of the mass. Interestingly, at 1-week follow-up visit we observed a visible reduction in the size of the lesion (Fig. 2). The histologic examination revealed proliferation of not-cohesive scattered cells with large-sized nuclei and prominent nucleoli. Immunohistochemistry analysis showed positivity for neuronspecific enolase, cytokeratin AE1/AE3, chromogranin, synaptophysin, pax5 and CD56, which confirmed the diagnosis of MCC (Fig. 3).
Computed tomography (CT) showed, upon the right palpebral region, a 41 mm├Ś16 mm mass with thickening of cutis and subcutaneous tissues from the internal canthus to the external canthus of the right eye and with involvement of the lateral rectus and superior rectus muscles. Both lymphatic and distant metastases were not observed. At the time of CT scan (nearly 15 days after the incisional biopsy) the tumor mass appeared to be further regressed (Fig. 4). Consequently, in agreement with our oncologists, neoadjuvant chemotherapeutic treatment with cisplatin (50 mg/m2) combined with etoposide (200 mg/m2) was undertaken, in order to reduce the volume of the lesion and try to avoid orbital exenteration.
Three cycles of chemotherapy were completed, but during the second cycle cisplatin had to be switched to carboplatin (400 mg/m2) due to an increase in creatininemia as a sign of kidney damage. The entire treatment lasted 3 months. As the neoadjuvant chemotherapy was over, CT examination demonstrated a marked reduction of the posterior-anterior diameter, whereas the transversal diameter was unchanged: from pre-chemotherapy 16 mm├Ś41 mm to post-chemotherapy 6 mm├Ś41 mm. However, the lateral rectus muscle appeared to be still infiltrated by the tumor. No other pathological findings were observed.
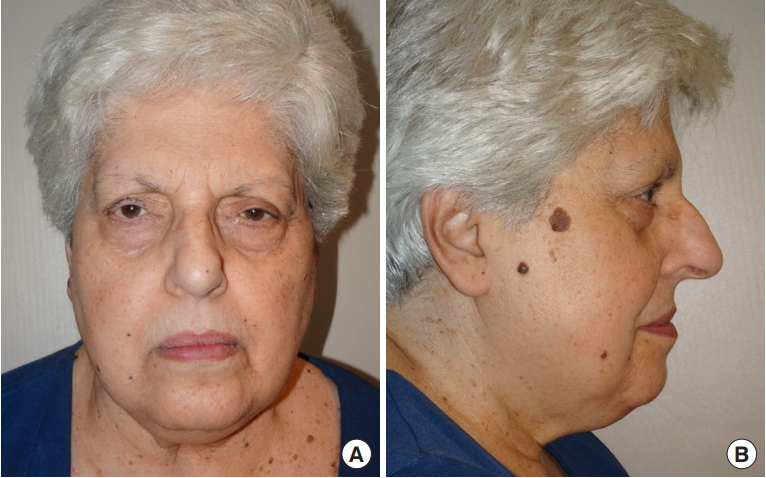
One month later, a preoperative magnetic resonance imaging (MRI) was requested. The MRI provided evidence of an irregular thickening of the right eyelid, associated with minimal ambiguous thickening of the aponeurosis and distal part of the levator palpebrae superioris muscle. No neoplastic invasion of the lateral rectus muscle of the eye was identifiable anymore, neither other pathological findings. At this time, the mass appeared to be macroscopically regressed (Fig. 5).
According to the result of the MRI we decided to proceed with the complete surgical excision of the lesion with 1,5-cm macroscopic lesion-free margins, which partially included the orbicularis muscle until the tarsal plane. Several biopsies of the skin, orbicularis muscle and levator palpebrae superioris muscle were performed. All the tissue samples resulted negative for neoplastic infiltration. Only evidence of inflammation was observable. No complications occurred during surgery. Three years later, no recurrence has been observed (Fig. 6).
MCC is a recognized neuroendocrine tumor, but there is still the need for further information about management and possible treatments. MCC often involves sun-exposed areas with 40% arising over face and neck [8]. Involvement of the eyelid occurs in nearly 10% of cases, with annual incidence of approximately 0.23 cases per 100,000 persons [9]. The rate of recurrence and mortality of eyelid MCC is lower than MCC that arises elsewhere, probably thanks to earlier detection [9]. Overall mortality rate is 28% at 2 years, which makes MCC even more lethal than melanoma [7]. Recurrence occurs within 2 years since removal of the primary tumor in 90% of cases, averagely by 8 months [7].
Clinically, MCC of the eyelid may mimic lymphoma, sebaceous carcinoma, or other primary tumors such as basal cell carcinoma (BCC), squamous cell carcinoma (SCC), adult neuroblastoma and amelanotic malignant melanoma, angiosarcoma, adnexal tumors, while metastatic neuroendocrine carcinoma and carcinoid tumor are very rare in the periocular region [7]. MCC may also simulate lesions such as keratoacanthoma and chalazion [8]. As it occurred in our case, other authors reported some cases of MCC misdiagnosed as chalazion and they all have in common lack of response to antibiotic therapy [5,10].
MCCs are usually recognized within 6 months from the time they first appear, due to the rapid growth of the mass, which is in contrast with other neoplasms such as BCC, SCC, sebaceous gland carcinoma, in which clinical signs may develop in more than 1 year before examination [5]. According to the more recent TNM staging system that has been published in 2017 by the American Joint Committee on Cancer [11], our case is classified as a stage II-B, meaning T4N0M0.
Although surgery remains the first line treatment for primary tumors, MCC has been observed to exhibit sensitivity to chemotherapeutic agents, especially if polychemotherapy (platinum-based antineoplastic associated with etoposide) is undertaken. Chemotherapy as a primary treatment has been proven to be acceptable in patients unable to undergo surgery [12].
Poulsen et al. [13] showed a locoregional control rate of 71% with only chemoradiotherapy, however in our case the surprising regression of the tumor and the excellent initial response to chemotherapy discouraged the association of radiotherapy, which would have carried a high risk of ophthalmic complications due to the neoplasm location. Some of the most common radiotherapic ophthalmic complications of upper eyelid include: xerophthalmia, scarring, chronic pain, corneal ulceration, keratinization of the palpebral conjunctiva, chronic conjunctivitis, entropion, ectropion. In addition, late radiotherapy effects may conceal or mimic recurrent malignancy [14].
The peculiarity of our case is that after biopsy, clinical spontaneous partial regression has occurred. However, a neoadjuvant chemotherapy was started because of involvement of the lateral rectus and superior rectus muscles of the eye. Although cases of post-biopsy spontaneous regression of MCC have been reported in literature [15,16], we are unable to state with certainty what led to lack of neoplastic cells in the area affected by the tumor and no relapse at 36-month follow-up. Probably both inflammatory response to biopsy and chemotherapy contributed to the tumor regression.
Preoperative neoadjuvant chemotherapy is usually reserved to patients with nodal or distant metastatic disease, however in our case we successfully started neoadjuvant chemotherapy in a locally advanced MCC in absence of lymphatic or metastatic disease, in order to avoid the excision of the eye bulb. To the best of our knowledge this is the first report about the use of neoadjuvant chemotherapy in MCC of the eyelid at II-B stage (T4N0M0). Other reports about the use of neoadjuvant chemotherapy in MCC include patients affected by not locally limited MCCs with nodal involvement [17,18].
According to our experience, neoadjuvant chemotherapy may be prescribed, in selected cases, also in locally advanced MCC, even in absence of lymphatic or distant metastasis, in order to avoid invasive surgical treatment. In our case, an immediate surgical approach after diagnosis would have led to the exenteration orbitae. Although we can not exclude that the tumor healed exclusively thanks to spontaneous regression after the incisional biopsy, our approach allowed the maintenance of the eye bulb and extrinsic eyeŌĆÖs muscles. In addition, a good functional and aesthetic result was achieved, with no recurrence of the tumor at 3-year follow-up. Probably the combination of the inflammatory cascade due to the incisional biopsy and neoadjuvant chemotherapy led to the regression of the tumor.
Moreover, it is critical to include MCC between the differential diagnoses in case of absence of response to treatment of a lesion initially recognized as chalazion, especially in advanced age patients.
Notes
Fig.┬Ā1.
Initial presentation, with tumor mass causing mechanical ptosis, in frontal (A) and lateral (B) views.
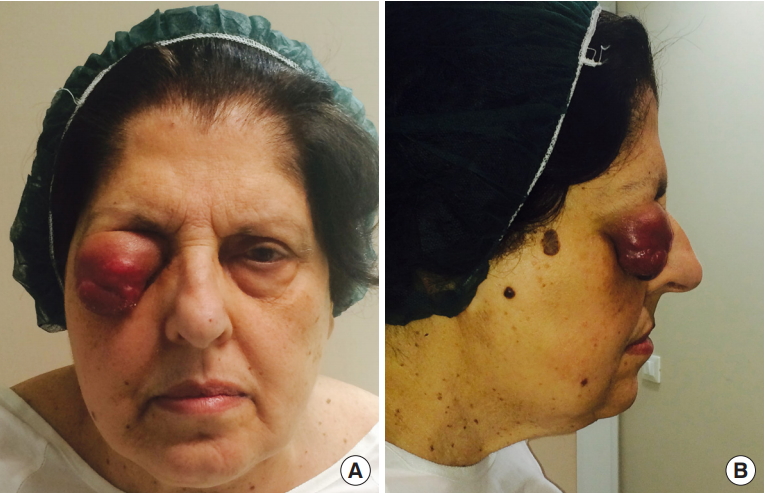
Fig.┬Ā2.
PatientŌĆÖs photograph 1 week after the incisional biopsy showing initial regression of the tumor mass.
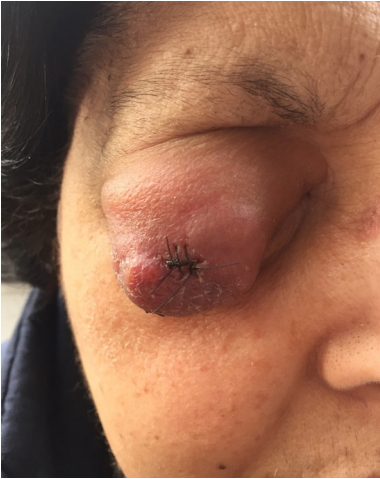
Fig.┬Ā3.
Tumor histology featured by scattered cells with large-sized nuclei and prominent nucleoli (H&E, ├Ś10).

REFERENCES
2. Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008;319:1096-100.



3. Kivela T, Tarkkanen A. The Merkel cell and associated neoplasms in the eyelids and periocular region. Surv Ophthalmol 1990;35:171-87.


4. Albores-Saavedra J, Batich K, Chable-Montero F, Sagy N, Schwartz AM, Henson DE. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol 2010;37:20-7.


5. Missotten GS, de Wolff-Rouendaal D, de Keizer RJ. Merkel cell carcinoma of the eyelid review of the literature and report of patients with Merkel cell carcinoma showing spontaneous regression. Ophthalmology 2008;115:195-201.


6. Lemos BD, Storer BE, Iyer JG, Phillips JL, Bichakjian CK, Fang LC, et al. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5823 cases as the basis of the first consensus staging system. J Am Acad Dermatol 2010;63:751-61.



7. Senchenkov A, Moran SL. Merkel cell carcinoma: diagnosis, management, and outcomes. Plast Reconstr Surg 2013;131:771e-778e.


8. Medina-Franco H, Urist MM, Fiveash J, Heslin MJ, Bland KI, Beenken SW. Multimodality treatment of Merkel cell carcinoma: case series and literature review of 1024 cases. Ann Surg Oncol 2001;8:204-8.


9. Herbert HM, Sun MT, Selva D, Fernando B, Saleh GM, Beaconsfield M, et al. Merkel cell carcinoma of the eyelid: management and prognosis. JAMA Ophthalmol 2014;132:197-204.


10. Rawlings NG, Brownstein S, Jordan DR. Merkel cell carcinoma masquerading as a chalazion. Can J Ophthalmol 2007;42:469-70.


11. American Cancer Society. Merkel cell carcinoma stages [Internet]. Atlanta, GA: American Cancer Society; 2018;[cited 2019 Feb 15]. Available from: https://www.cancer.org/cancer/merkel-cell-skin-cancer/detection-diagnosis-staging/staging.html.
12. Samonis G, Mantadakis E, Kononas TC, Rigatos SK, Stathopoulos GP. Merkel cell carcinoma: a case series of twelve patients and review of the literature. Anticancer Res 2001;21:4173-7.

13. Poulsen M, Rischin D, Walpole E, Harvey J, Mackintosh J, Ainslie J, et al. High-risk Merkel cell carcinoma of the skin treated with synchronous carboplatin/etoposide and radiation: a Trans-Tasman Radiation Oncology Group Study. TROG 96:07. J Clin Oncol 2003;21:4173-6.

14. Jeganathan VS, Wirth A, MacManus MP. Ocular risks from orbital and periorbital radiation therapy: a critical review. Int J Radiat Oncol Biol Phys 2011;79:650-9.


15. Ahmadi Moghaddam P, Cornejo KM, Hutchinson L, Tomaszewicz K, Dresser K, Deng A, et al. Complete spontaneous regression of merkel cell carcinoma after biopsy: a case report and review of the literature. Am J Dermatopathol 2016;38:e154. -8.


16. Karkos PD, Sastry A, Hampal S, Al-Jafari M. Spontaneous regression of Merkel cell carcinoma of the nose. Head Neck 2010;32:411-4.


- TOOLS
-
METRICS

-
- 6 Crossref
- Scopus
- 5,307 View
- 94 Download
- Related articles in ACFS