Acellular dermal matrix (Insuregraf) in the prevention of Frey’s syndrome and surgical site depression after parotidectomy
Article information
Abstract
Background
Parotidectomy is the treatment of choice in many parotid tumors. Due to the extensive nature of the procedure, unfavorable complications such as gustatory sweating, surgical site depression are common. Various techniques using fascia, muscle or AlloDerm have been developed but debate still remains regarding its availability and affordability. We applied a newly developed acellular dermal matrix (Insuregraf) to the parotidectomy field to act as a physical barrier and to provide adequate filling effect for prevention of functional and aesthetic complications.
Methods
From March 2010 to March 2017, 30 patients with parotid tumors underwent superficial parotidectomy. Twenty patients underwent only superficial parotidectomy. Ten patients had Insuregraf applied to the surgical site after superficial parotidectomy. We evaluated the incidence of Frey’s syndrome, surgical site depression, and patient satisfaction rate in both groups.
Results
The incidence of Frey’s syndrome was lower in the Insuregraf group (0 vs. 2). Surgical site depression was also lower in the Insuregraf group (2 vs. 20). Satisfaction score for facial contour in Insuregraf group was 9.2 out of 10, which was comparable to 6.2 out of 10 in the control group.
Conclusion
Application of Insuregraf after superficial parotidectomy is an effective surgical procedure to prevent complications such as Frey’s syndrome and surgical site depression. This technique is affordable and safe with no immune reactions. Above all this surgical method should be considered as an option for patients who are concerned about the contour of the face after surgery.
INTRODUCTION
The parotid tumors account for about 3% of the head and neck tumors and account for 80% of the tumors in the salivary gland [1,2]. Due to its benign but extensive nature, it is usually treated by removing the parotid gland. Due to the encasement of the facial nerve inside the parotid gland, prevention of the facial nerve was the most important focus during the surgical procedure. It is a fact that other complications such as Frey’s syndrome, surgical site depression, ear sensory abnormality, scar, pain, and dry mouth have been relatively overlooked [3]. The most common complication is Frey’s syndrome and depression of the surgical site [4]. Frey’s syndrome is also known as gustatory sweating or auriculotemporal nerve syndrome. It is characterized by sweating and flushing of the parotid area skin due to the aberrant nerve connections made between nerves of the skin and the remaining parotid gland [5]. Since the first report of Duphenix in 1757, many publications have reported an incidence of 11% to 95% [6,7]. In 1923, Frey [5] reported that the occurrence of Frey’s syndrome was associated with auriculotemporal nerve. In 1927, Andre Thomas suggested that parasympathetic nerves grow abnormally in the sweat glands and blood vessels of the skin covering the parotid gland, causing Frey’s syndrome. Depression of the surgical site after parotidectomy results in facial asymmetry and occurs in most patients [4,8]. Frey’s syndrome and skin depression after parotidectomy are very common and cause significant aesthetic and quality of life issues. To prevent such complications, many surgical methods have been introduced to prevent abnormal nerve connection. This is usually achieved by placing a physical barrier such as fascia, muscle or artificial dermal matrix between the parotid gland and the skin. Most widely reported method is the placement of AlloDerm sheet over the dissected plane. However, its accessibility is limited due to high cost and limited amount of source from cadaveric skin.
Insuregraf (SK Bioland, Cheonan, Korea) is a highly-porous, native dermal matrix consisting of 100% of type I collagen derived from porcine skin which can provide a scaffold structure for an organized colonization by dermal cells and facilitate the reconstruction of soft tissue defect. There have been a few reports on utilizing Insuregraf in burn victims, which showed excellent results in terms of dermal layer organization, skin thickness, and skin elasticity. In this regard, we hypothesized that Insuregraf could simultaneously act as a physical barrier and provide adequate tissue thickness after parotidectomy. In this article, we report our experience of utilizing Insuregraf as an alternative material for prevention of common complications following superficial parotidectomy.
METHODS
Study design and patient demographics
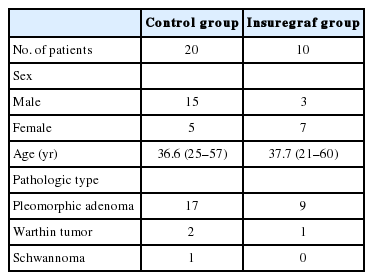
This study was approved by the Institutional Review Board of Catholic University of Korea (IRB No. UC18RESI0063). This study included 30 patients who underwent superficial parotidectomy from March 2010 to March 2017. The patients were then randomly divided into two groups: 20 patients (control group) underwent superficial parotidectomy only and 10 patients (Insuregraf group) received superficial parotidectomy and Insuregraf was placed before skin closure.
Surgical technique
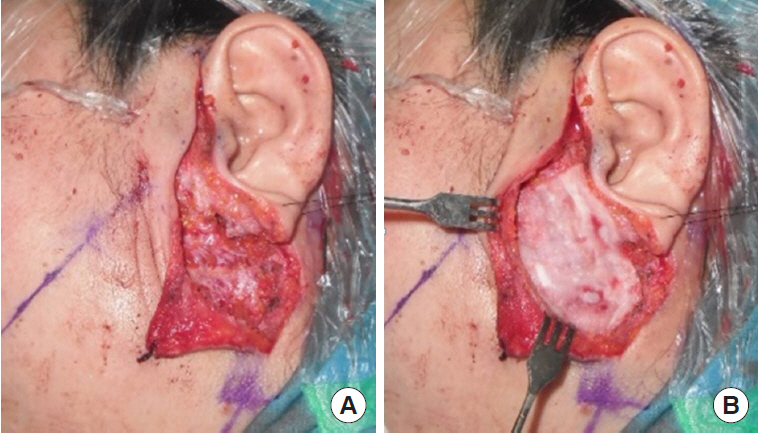
All operations were performed by a single surgeon (SNJ) and the operative procedure was uniform in all cases of this study, which consisted of superficial parotidectomy. A lazy-S shaped Blair incision was applied from the preauricular area to the proximal one-third of mandibular body. Skin flap was elevated to the anterior margin of the masseter muscle. In the control group, skin flap was directly closed after the excision of the superficial parotid gland. For the Insuregraf group, a matrix sheet of Insuregraf was placed over the superficial parotidectomy field (Fig. 1). Insuregraf was trimmed to fit the depressed area and was 4×8 cm in size on average. Insuregraf was fixed to the surrounding tissues using Tisseel (Baxter, Deerfield, IL, USA) for greater stability and the skin flap was closed (Fig. 2). A small sized negative suction drain was placed inside the surgical field in both groups for 3 to 7 days for prevention of hematoma.
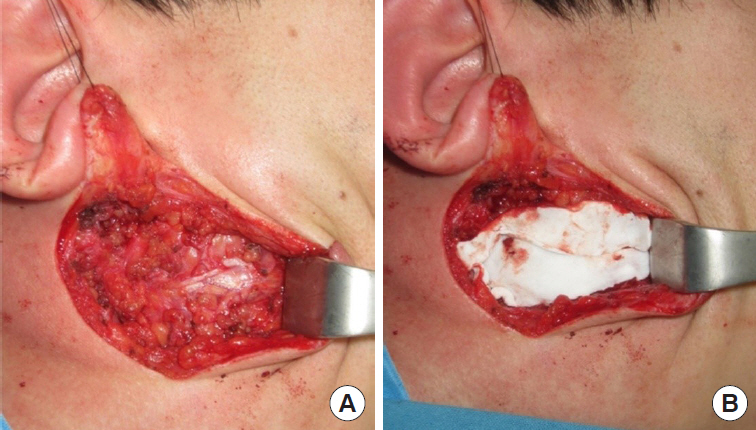
A 21-year-old man with a pleomorphic adenoma on his right parotid gland area for 6 months. (A) After superficial parotidectomy. (B) Insertion of Insuregraf after parotidectomy.
Evaluation of surgical outcome
The symptoms of Frey’s syndrome and facial asymmetry due to surgical site depression were evaluated at 18 months postoperatively. A different physician uninvolved in the surgery was invited to evaluate the presence of surgical site depression and Frey’s syndrome. Patient overall satisfaction for facial contour was evaluated with a 10-point scale questionnaire.
Statistical analysis
Incidence of Frey’s syndrome and surgical site depression was compared with Fisher exact test. The mean satisfaction score was analyzed with Mann-Whitney test.
RESULTS
Of the 30 patients, male to female ratio was 18 to 12 (60% vs. 40%). The mean age was 37 years (range, 21–60 years). Of these, 26 patients had pleomorphic adenoma, three patients had Warthin tumor and one patient was diagnosed with schwannoma (Table 1).
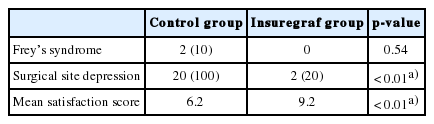
All 30 patients completed the study. No cases of facial nerve damage, immune reaction to the graft material, or salivary fistula was observed in either group. Frey’s syndrome did not occur (0%) in the Insuregraf group. Two patients (10%) experienced Frey’s syndrome in the control group (Fisher exact test, p=0.54). Surgical site depression occurred in two patients (20%) in the Insuregraf group and 20 patients (100%) in the control group (Fisher exact test, p<0.01). The mean satisfaction score for facial contours was 9.2 out of 10 in the Insuregraf group and 6.2 out of 10 in the control group, showing a significantly higher score in the Insuregraf group (Mann-Whitney test, p<0.01) (Table 2).
DISCUSSION
Preventing Frey’s syndrome and surgical site depression after parotidectomy procedure has been a longtime concern. Various methods have been reported to prevent complications after parotidectomy. Autogenous vascularized flaps such as the sternocleidomastoid (SCM) muscle flap, the superficial musculoaponeurotic system (SMAS) flap, the temporoparietal flap, and the platysma flap are the procedures that have been used before artificial dermal matrix products gained popularity. Although widely supported, each strategy retains its own drawbacks. SCM muscle flap can provide bulk and is suitable for cases where severe depression is expected. However, possible injury to the spinal accessory nerve, and delayed detection of tumor recurrence under the thick muscle bulk are major drawbacks [9-11]. Temporoparietal fascia is a versatile flap arising from the superficial temporal artery. This flap is wide enough to completely cover the exposed parotid gland and facial nerve. However, additional scalp incision followed by scar alopecia, hollowness of the temporal area is a major limitation of this technique [12-14]. The SMAS flap, the most widely used, can be used as superior rotation, posterior advancement or plication as well as redraping method depending on the location and size of the defect [15]. Surgical procedure is relatively simple because the flap can be elevated in the same surgical field [16]. However, it is impossible to employ this technique for extensive tumors, which sometimes requires en bloc removal of the tumor including the SMAS tissue [17]. SMAS flap is thinner than SCM muscle flap and is not suitable for large defect sites [13-17].
The use of autogenous non-vascularized tissues such as fascia lata, free fat or dermofat has been attempted for decades to cover the defect site after parotidectomy. However, no large-scale studies using this method have been published.
The fat graft has a similar texture to the parotid gland, which is a good way to supplement the defect after parotidectomy [18]. Moreover, this method is easy to handle and the donor site scar is small [18-20]. However, the rate of absorption varies from 25% up to 80%, and complications such as fat liquefaction, seroma and sialocele may occur [21-23]. To overcome the problem of reabsorption after fat graft, transplantation with dermis or overcorrected 15% to 30% has been used [13,19,20]. However, dermal fat grafts also vary in absorption rate and can cause inflammation due to dregs of dermis [19].
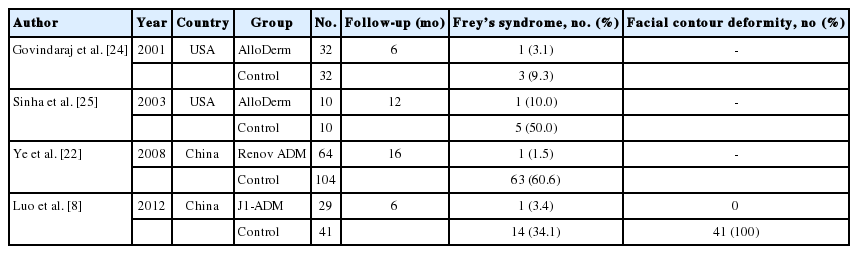
Recently, various allogenic implants have been introduced to fill the parotid defect [8,21-25]. These methods are easy, have a short operation time, and do not cause donor problems [8,25]. AlloDerm is a commonly used acellular dermal matrix (ADM) obtained from cadaveric skin and it has been used increasingly in the last decade. Previous studies have reported that ADM materials, including AlloDerm, are effective in lowering the incidence of Frey’s syndrome and bringing good cosmetic results (Table 3). Despite its excellent characteristics, the material is very expensive and its supply is limited. Furthermore, it requires refrigeration for storage and requires over 30 minutes of hydration before implantation.
Insuregraf, the material we incorporated in this study, is a three-dimensional matrix composed of porcine collagen, which shows high similarity to human dermis. In contrast with Allo-Derm, Insuregraf is sufficient in its supply due to abundance of porcine dermis material. Furthermore, it does not require prehydration. The native structurally intact collagen serves as an essential component of the new extracellular matrix for the migration of cells and vascularization. The programmed thickness of Insuregraf allows initial supply to the graft by diffusion and rapid vascularization. According to our results, use of Insuregraf exhibited excellent surgical outcomes compared to the control group, in terms of surgical site depression, incidence of Frey’s syndrome, and patient satisfaction. However, difference in the prevalence of Frey’s syndrome failed to show any significant results. However, considering the structural property and chemical composition of Insuregraf, we assume that it has sufficient potential ability to yield significant differences in a large group study. Therefore, we think that Insuregraf can be a feasible choice for preventing common complications after parotidectomy. Compared to other methods, this material shows advantage in its convenience in storage, direct usage, and low cost.
Due to our small sample size and limited parameters for evaluation, further studies with larger sample scale are definitely needed. A head-to-head comparison study with AlloDerm can be undertaken for future investigation.
Insuregraf, a porcine dermal matrix sheet, improved surgical outcomes compared with control. It reduced the occurrence of Frey’s syndrome and surgical site depression. Patient overall satisfaction scores for postsurgical cosmesis were higher. We think that Insuregraf can be an alternative method to autologous reconstruction in terms of shorter surgical time and low surgical morbidity. Compared to AlloDerm, Insuregraf is easily affordable, and much more convenient to use in terms of storage and implantation. Therefore, we believe that Insuregraf can be an efficient option for better functional and esthetic outcomes after parotidectomy procedures.
Notes
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was approved by the Institutional Review Board of the Catholic University of Korea (IRB No. UC18RESI0063) and performed in accordance with the principles of the Declaration of Helsinki. Written informed consents were obtained.
Patient consent
The patients provided written informed consent for the publication and the use of their images.