Oroantral fistula after a zygomaticomaxillary complex fracture
Article information
Abstract
Zygomaticomaxillary complex (ZMC) fractures account for a substantial proportion of trauma cases. The most frequent complications of maxillofacial fracture treatment are infections and soft tissue flap dehiscence. Postoperative infections nearly always resolve in response to oral antibiotics and local wound care. However, a significant infection can cause a permanent fistula. A 52-year-old man visited our clinic to treat an oroantral fistula (OAF), which was a late complication of a ZMC fracture. Postoperatively, the oral suture site dehisced, exposing the absorbable plate. However, he did not seek treatment. After 5 years, an OAF formed with a 2.0× 2.0 cm bony defect on the left maxilla. We completely excised the OAF, harvested a piece of corticocancellous bone from the iliac crest, inserted the harvested bone into the defect, and covered the soft tissue defect with a buccal mucosal transposition flap. Although it is necessary to excise OAFs, the failure rate is higher for large OAFs (> 5 mm in diameter) because of the extensive defect in the underlying bone that supports the overlying flap. Inappropriate management of postoperative wounds after a ZMC fracture can lead to disastrous outcomes, as in this case. Therefore, proper postoperative treatment and follow-up are essential.
INTRODUCTION
Zygomaticomaxillary complex (ZMC) fractures occur in a substantial proportion of trauma cases. Postoperative infections take place in 1.5% of fracture cases, and nearly always resolve in response to oral antibiotics [1]. However, significant infections can cause early complications, such as exposure of the reduction site, and late complications, such as permanent fistula [1,2].
Oroantral communication (OAC) is a pathological connection between the oral cavity and the maxillary sinus. The most common cause of OAC is extraction of the posterior maxillary teeth. Other causes include maxillary sinus cysts, benign and malignant tumors, and trauma. In rare cases, inappropriate postoperative management can also cause OAC [2]. Early treatment of OAC is important to prevent the formation of an oroantral fistula (OAF) and chronic maxillary sinusitis [3]. In this case report, a case of OAF as a late complication after the reduction of a ZMC fracture is presented and the surgical prognosis is discussed.
CASE REPORT
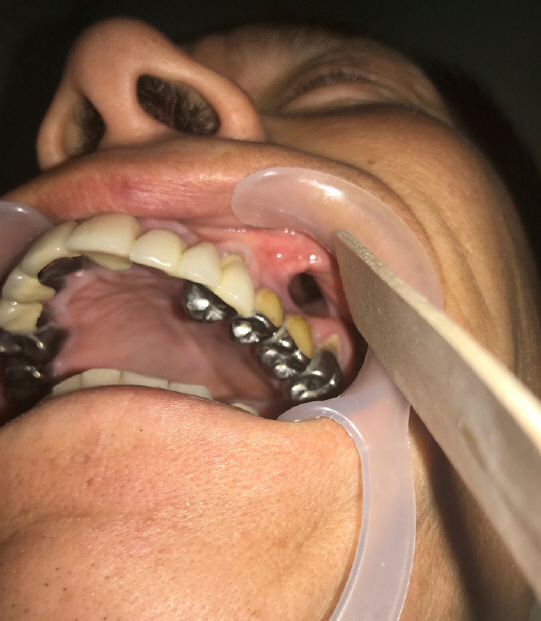
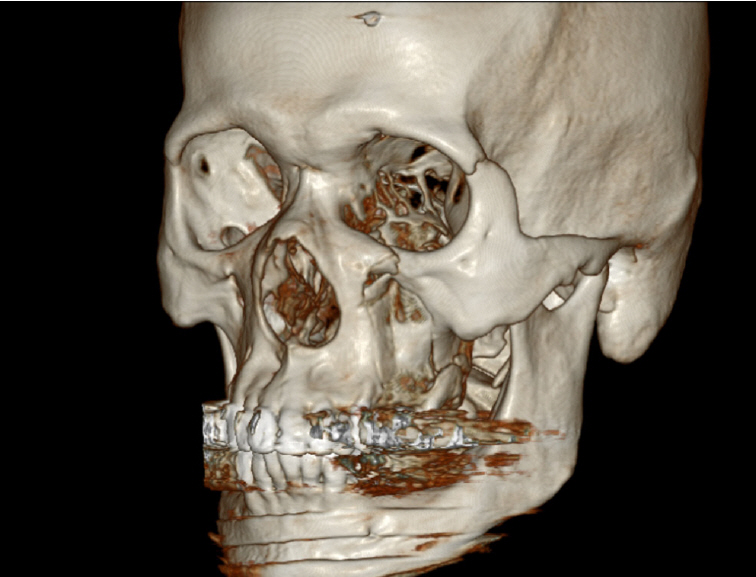
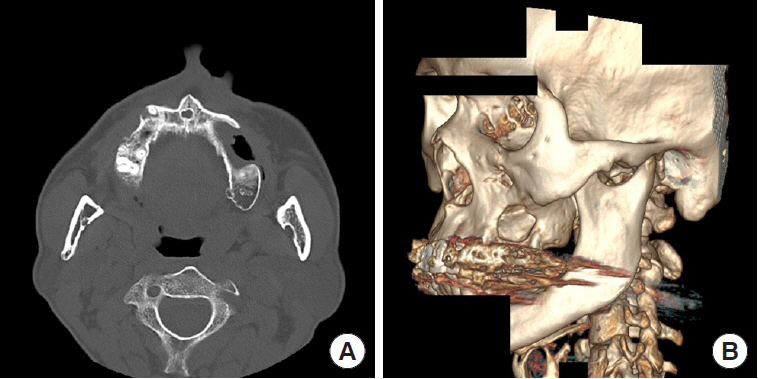
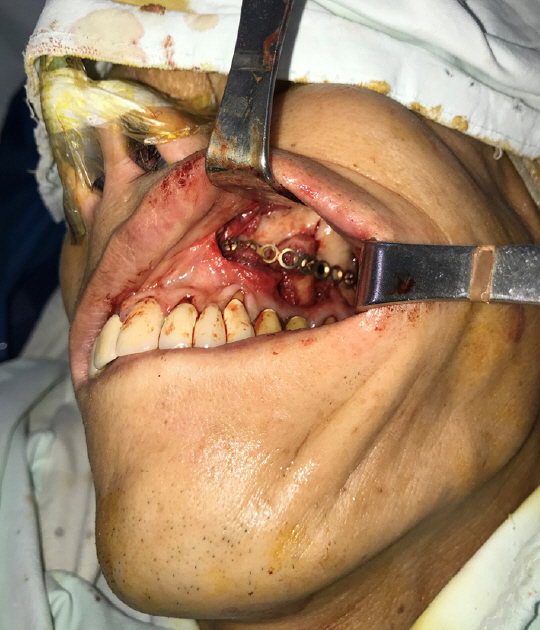
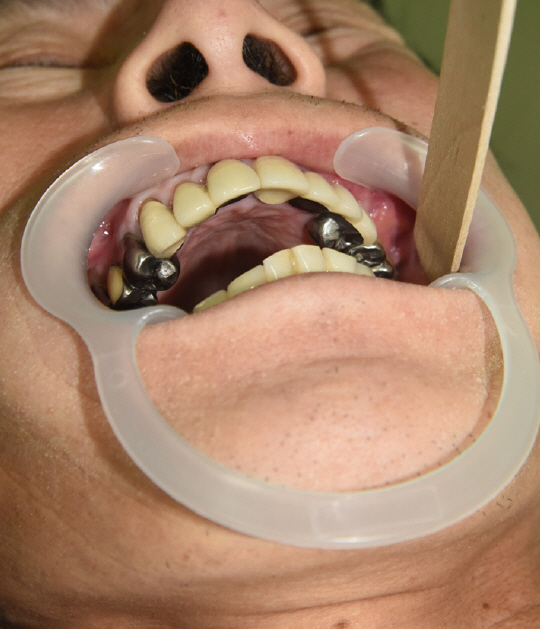
A 52-year-old man visited Soonchunhyang University Gumi Hospital to treat an OAF of the left maxilla (Fig. 1). He had a history of ZMC fracture and had undergone open reduction surgery with an absorbable plate 5 years previously (Fig. 2). Postoperatively, the oral suture site dehisced, exposing the absorbable plate. The patient did not seek treatment for the disrupted wound despite several episodes of sinusitis and inflammation of the oral cavity. After 5 years, an OAF opened and became filled with dirty granulation tissue. Food items such as rice frequently entered the gingival cavity, causing the patient to experience discomfort, which led him to visit our clinic. For diagnosis, three-dimensional computed tomography scans and radiographic images were obtained. Radiological findings showed a 2.0×2.0 cm bony defect on the alveolar bone along with the OAF (Fig. 3). Excision of the OAF and coverage of the bony defect were indicated.
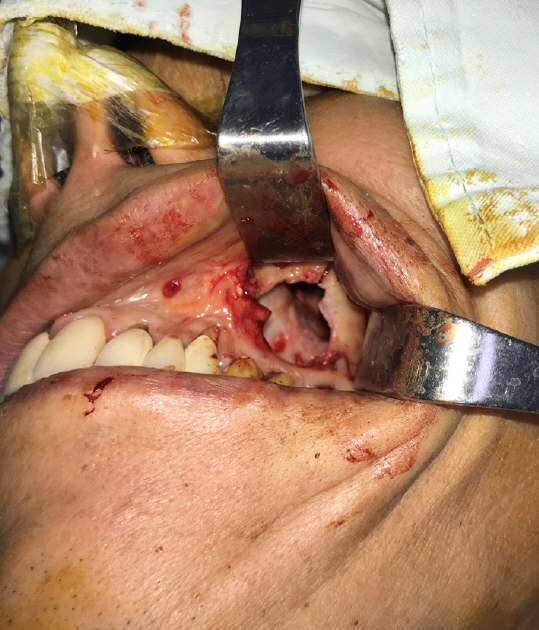
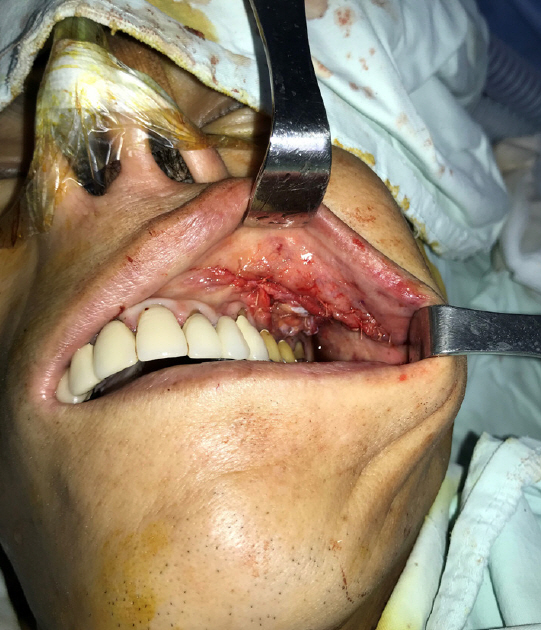
Intraoperatively, the OAF was marked with dye to visualize the boundary of the affected tissue. To excise the fistula, a circular incision was made around the OAF with a 2-mm margin, and the epithelial tract and any inflammatory tissue (i.e., granulation tissue) within the opening were completely excised. During the removal of the granulation tissue, we found rice in the maxillary sinus. The size of the bony defect was 2.0×2.0 cm (Fig. 4). A piece of corticocancellous bone from the iliac crest was harvested, inserted into the bony defect, and fixed with a straight 12-hole miniplate (Synthes, Paoli, PA, USA) and screws (6 mm; Synthes) (Fig. 5). The soft tissue defect was covered with an elevated transposition flap (Fig. 6). A trapezoidal buccal mucoperiosteal flap was reflected from the alveolar process and lateral wall of the maxilla, and released to the gingivolabial sulcus.
Ceftriaxone and metronidazole were intravenously administered for 10 days postoperatively, along with analgesics. The patient was instructed to avoid allowing hard food to contact the operated side for 2 weeks, tongue rolling over the suture line for 1 week, and nose blowing or sneezing with a closed mouth for 2 weeks.
After 4 weeks, the patient had no complaints of pain at the flap surgery and donor sites. The buccal mucosal flap was well healed, with no evidence of the defect at the donor site. At 8 weeks, he reported no symptoms at the graft site, and the symmetrical hip flexor strength remained unchanged. After 1 year, the OAF was completely healed and no other complications occurred (Fig. 7).
DISCUSSION
OAC is a complication that can occur after extraction of the upper posterior maxillary teeth accompanied by osteotomy [4]. Other causes of OAC include maxillary cysts, benign or malignant tumors, and trauma [5]. Inappropriate management of postoperative wounds can also cause OAC or OAF.
An OAC must be closed to prevent sinusitis caused by contamination of the maxillary sinus from the oral cavity via communication of the squamous epithelium of the oral cavity with the pseudostratified columnar ciliated respiratory cells of the maxillary sinus [6]. The best treatment for OAC is immediate surgical repair. If not properly detected, a large OAC may develop into an OAF.
An OAF is a pathological epithelialized communication between the oral cavity and the maxillary sinus. Transmission of substances from the oral cavity to the sinus causes maxillary sinusitis. Epithelialization of the fistulous tract and osteitis of the surrounding bony margins can inhibit spontaneous healing, resulting in chronic fistula formation [7]. Immediate plastic surgery to treat OAF is 95% effective, whereas delayed surgery has a success rate of only 67% [7].
A small OAC measuring 1–2 mm in diameter (without full epithelialization and sinus infection) can heal spontaneously after blood clot formation. However, OACs with a larger size or a duration of >3 weeks require early surgical treatment. Over a period of 3 weeks, fatty tissue transforms into granulation tissue and epithelializes [8]. OACs of >5 mm require the use of flaps for closure [2].
The risk factors of failure of surgical intervention for OAC or OAF include the size of the fistula, sinus infection, osteitis of the margins, epithelialization of the fistulous tract, and excessive tension on the flap, which impairs blood supply. The most common cause of chronic OAF and repair failure is ineffective treatment of sinusitis. In such cases, endoscopic sinus surgery or a Caldwell-Luc procedure may be required [9].
A buccal fat pad can be used in fistulas with a diameter of 8–20 mm. Borgonovo et al. [2] concluded that buccal advancement flaps alone are an ideal treatment for small OAFs. In large OAFs, buccal flaps must be combined with palatal flaps. The limitations of buccal flaps include lowering of the vestibulum.
In large OAFs (>5 mm in diameter), the failure rate increases because of the size of the defect in the underlying bone. According to the extensive literature on this topic, autologous bone grafts are the preferred treatment for bone defects.
An auricular cartilage graft is another option for closure of the OAF. Such grafts have the advantages of being biocompatible, easy to harvest, and non-resorbable; however, a disadvantage of this technique is the risk of defect formation at the donor site [10].
ElShourbagy et al. [11] achieved successful closure of an OAC using Fisiograft bone filler. The combined use of sponge-form Fisiograft together with a platelet-rich fibrin membrane was found to be a simple, easy, and effective technique for OAC closure.
An iliac bone graft was preferred for the case described herein because the patient’s bony defect was more suitable for a fully completed material. The iliac bone is larger and thicker than the auricular cartilage and is easy to handle. Moreover, the iliac bone is autogenous, which makes it more biocompatible than Fisiograft. In this case of a late infection, autogenous substances were preferred.
This rare case of OAF was caused by inappropriate postoperative wound management in conjunction with a late complication of a ZMC fracture. The patient had a normal alveolar bone and no other bony abnormality at the ZMC fracture site. Presumably, after the oral suture site was disrupted and the absorbable plate was exposed, food interfered with the closure of the OAF. Epithelization of the fistula tract and growth of dirty granulation tissue followed, further disrupting healing. Over several years, external factors such as food may have caused inflammation and osteolysis in the alveolar bone. Alternatively, chronic inflammation of the maxillary sinus may have been responsible for osteolysis, or the patient’s edentulous condition may have stimulated bone defect formation.
The exact cause of the alveolar defect remains unknown. However, inappropriate management of postoperative wounds after repair of a ZMC fracture can lead to serious sequelae, such as the large bony defect with OAF observed in this case, which required an additional surgical intervention that included a buccal transposition flap and a bone graft. Proper management, such as asking patients to gargle with antibiotic solutions and encouraging them to follow proper postoperative care after a ZMC fracture, should be combined with careful inspections during postoperative visits to prevent later complications.
Notes
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was performed in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained.
Patient consent
The patient provided written informed consent for the publication and the use of his images.