|
 |
- Search
| Arch Craniofac Surg > Volume 20(6); 2019 > Article |
|
Abstract
Renal cell carcinoma (RCC) represents 2% to 3% of human cancers and is aggressive, with metastatic capability. The frequent metastatic sites are lung, bone, and liver. Reports of RCC metastatic to skin, and especially scalp are rare. Here we present an 83-year-old woman who was diagnosed with RCC 19 years prior and had a metastatic scalp lesion. An 83-year-old woman presented with a red-to-purple, protruding lesion at the right parietotemporal area. Twenty-three years ago, a right renal mass was incidentally discovered on ultrasound through a routine medical examination. She underwent right nephrectomy for RCC 4 years later. Five months after nephrectomy, new lung nodules were observed. Fifteen years after nephrectomy, metastatic lesions were found in the pelvic bone. She visited dermatology department for evaluation of the new scalp lesion, a year before she first visited our department. Despite chemotherapy, the mass was gradually enlarged. She consulted the plastic surgery department for management of the metastatic RCC was successfully treated with total excision including a 1-cm safety margin, local flap, and STSG coverage. Complete healing was observed, without evidence of recurrence during a 7-month followup. Metastases to the skin are rare, but must be kept in mind because of its high metastatic ability and poor prognosis.
Renal cell carcinoma (RCC) is a cancer that originates in the proximal tubule epithelium of the kidney. RCC makes up the majority of kidney tumors, accounting for 80% to 90% of all malignant kidney cancers and represents 2% to 3% of human [1]. The most common symptoms of a so-called Triad are hematuria, abdominal pain, and a palpable flank mass, though fewer than 10% of the patients present with all three [2]. RCC also presents with nonspecific symptoms such as weight loss, fever, and hypertension, as well as left sided varicocele in males. Anemia, as a result of erythropoietin suppression, can also occur. In less than 5%, paraneoplastic syndromes result in hypercalcemia or erythrocytosis [1]. More than 70% of RCCs are found incidentally on imaging studies such as regular health medical examination. At the time of diagnosis, metastatic disease is present in approximately one-third of the patients. RCC is aggressive, with metastatic capability. RCC is known to primarily metastasize in to lymph nodes, bone, contralateral kidneys, lungs, liver, adrenal glands, brain, and less frequently to skin [3]. Reports of RCC metastatic to the skin, especially scalp, are rare. Here we present a female aged 83 years who was diagnosed with RCC 19 years prior and now had a metastatic scalp lesion.
An 83-year-old woman presented with a red-to-purple, protruding lesion measuring 3.0├Ś3.5 cm at the right parietotemporal area (Fig. 1). Twenty-three years ago, a right renal mass was incidentally discovered on ultrasound as part of a routine medical examination. She underwent right nephrectomy for RCC 4 years later. Pathology confirmed a 4├Ś3 cm Fuhrman grade IV (Robson stage I) RCC. Five months after nephrectomy, new right upper lobe and bilateral lobe lung nodules were observed on performing chest computed tomography (CT). However, she did well for the next 15 years without any treatment. Fifteen years after nephrectomy, metastatic nodules were found in the both lungs, left iliac bone, and L4 vertebral body on positron emission tomography scans. She underwent target therapy with pazopanib for 2 years, and everolimus for 2 months. A year before she first visited our department, she visited dermatology department for evaluation of the newly occurred scalp mass and underwent punch-biopsy. Biopsy confirmed metastatic RCC. Despite chemotherapy on scalp lesion, the mass was gradually enlarged from 2.6├Ś1.7 cm to 2.5├Ś3.4 cm. Finally, she consulted plastic surgery department for management of the metastatic scalp RCC.
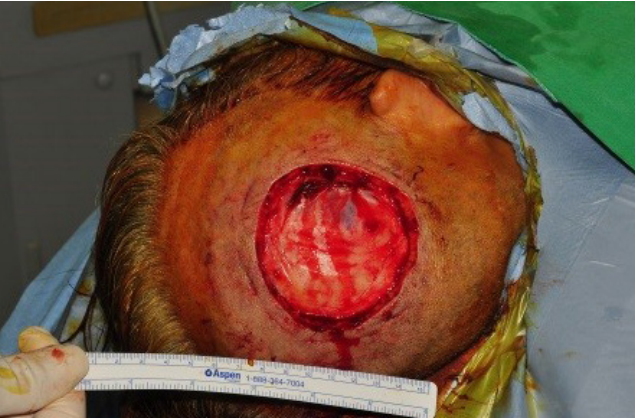
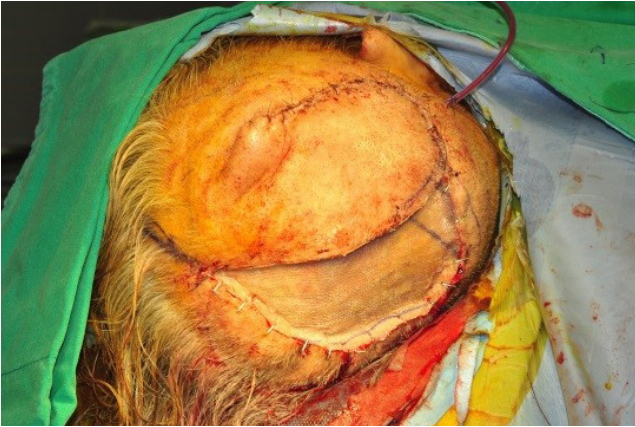
The patient was referred for excision of the mass. Under the general anesthesia, total excision with periosteum was performed, including a 1-cm safety margin (Figs. 2, 3). The excised mass examined microscopically by frozen and paraffin sections. The histopathological examination showed clear cells with round nuclei and abundant cytoplasm. The clear cells also appeared to form glandular or acinar structures, and have nuclear pleomorphism typically found in RCC (Fig. 4). All margins were cancer-free in frozen biopsy. The rotation flap was elevated and covered the defect. The split-thickness skin graft (STSG) was done to cover the newly formed raw surface due to rotational flap. The harvested STSG from the right lateral thigh was held on the defect (Fig. 5).
Complete healing of the excision site was observed, without evidence of recurrence during a 7-month follow-up (Fig. 6).
In the United State, 39,226 new cases of RCC and 10,662 deaths were reported in 2008. In the European Union, RCC accounts for roughly 3% of all cancers in men and 2% in women. In Korea, RCC accounted for 1.85% of the total cancer in 2012 [4]. Peak incidence occurs between fifth and seventh decade (mean 66 years), with higher incidence in men (2:1) [5].
Diagnosis is made by imaging, including ultrasound and abdominal CT. At recent, the incidence of RCC increased in conjunction with improvements in image device. More than 70% of RCCs are found incidentally on routine imaging. At the time of diagnosis, metastatic disease is present in approximately onethird. RCC is known to primarily metastasize in to lymph nodes, bone, contralateral kidneys, lungs, liver, adrenal glands, brain, and less frequently to skin. According to previous studies and reviews of RCC, metastatic RCC to the skin is rare. Less than 100 reports of RCC with metastasis to the skin and less than 20 with metastasis to scalp have been reported (Table 1) [1,5-12].
RCC spreads via various mechanisms including hematogenous dissemination, lymphatic extension, direct invasion from underlying tumor, and implantation from procedures. Among them, the hematogenous dissemination is considered the primary mechanism. The abundant venous circulations such as the prevertebral, vertebral and epidural system permit the tumor spread to the other sites. Tumor cells spread with less resistance through valveless veins. The route is renal vein to vena cava to right atrium to lung. Massive metastasis to the renal vein or vena cava by tumor emboli is a leading characteristic of RCC. Other routes are the spermatic (or ovarian) veins and the vertebral veins. Tumor cells spread to the pelvic organs such as ureter, urinary bladder, ovary, vagina and penis through the spermatic veins. The vertebral vein (or plexus of Batson) allows preferential localizations in the vertebral column, thyroid and central nervous system [6]. Retrograde flow from increased intraabdominal or intrathoracic pressure permits tumor emboli spread to the renal veins, the plexus of Batson, the vertebral veins, and to emissary scalp veins. In this way, RCC can metastasize in head, neck and skin, while the precise mechanisms are still unclear.
Nephrectomy is the treatment of choice for RCC without any metastasis. Nephrectomy offers 5-year survival rate of above 90% in patients with stage I RCC and about 40% in patients with stage II and III RCC [13]. When the tumor has already invaded the renal vein or the vena cava without distant metastasis, surgical removal can provide a chance of cure [5]. At present, there is no defined role for adjuvant therapy for localized RCC following partial or radical nephrectomy. The current standard of care involves serial imaging with CT, typically up to a span of 5 years [14].
Targeted therapy agents have received US Food and Drug Administration approval and are available in the first-line setting for metastatic RCC. RCC is resistant to radiation, traditional cytotoxic, or hormone therapy. Advances in the understanding of RCC biology and pathology resulted in the successful development of targeted therapy for RCC. For metastatic RCC, treatment consists of nephrectomy plus targeted therapy with a tyrosine kinase inhibitor (sunitinib), vascular endothelial growth factor inhibitor (bevacizumab), or the mammalian target of rapamycin (mTOR) inhibitor (everolimus). The standard of care is close observation although no standard guidelines exist, monitoring every 3 months with CT represents a reasonable practice. Patients with limited sites of metastatic RCC should consider metastasectomy. Symptomatic bone metastases should be evaluated for stereotactic radiation therapy. Brain metastases should be treated surgically or by stereotactic radiosurgery or whole-brain radiation therapy prior to systemic therapy [15].
In our case, the tumor invaded both lungs, multiple bony site, and the scalp, even after nephrectomy. She was treated with target therapy, including pazopanib and everolimus. Pazopanib is an orally administered angiogenesis inhibitor targeting the vascular endothelial growth factor receptor, platelet-derived growth factor receptor, and c-Kit. Pazopanib is under clinical development for the treatment of multiple tumor types and has demonstrated monotherapy activity in RCC. Everolimus is an orally administered mTOR inhibitor, a component of an intracellular signaling pathway that regulates cellular metabolism, growth, proliferation, and angiogenesis. Everolimus, a derivative of rapamycin, binds to an intracellular protein, FKBP-12, forming a complex that inhibits the mTOR serine-threonine kinase. Everolimus was found to prolong progression-free survival relative to placebo in metastatic RCC [16].
Differential diagnosis in our case included other cutaneous tumors such as sebaceous carcinoma, sweat gland tumor, Kaposi sarcoma, angiosarcoma and melanoma.
The prognosis with cutaneous metastases is poor. The survival range is from 10.2 months to 22 months, and the mean 5-year survival rate with a single cutaneous metastasis of RCC is 13% to 50%. The rate of patients with multiple lesions do not exceed 8% [3]. Patients with a long disease-free interval from diagnosis to metastasis have a longer survival rate [7]. In our case, despite poor prognosis, especially with multiple metastates, our patient is alive and well without recurrence after excision of the scalp lesion.
In conclusion, we report a case of cutaneous metastatic RCC to the scalp. Metastases to the skin are rare, but must be kept in mind because of its high metastatic ability and poor prognosis. Even patients after nephrectomy should take regular follow-up for monitoring metastases. It is also important to be able to recognize the clinical symptoms or signs of metastatic RCC, as they may be the first indication of underlying malignancy.
Notes
Fig.┬Ā1.
A red-to-purple, protruding lesion measuring 3.0├Ś3.5 cm was noted at the right parietotemporal area.
Fig.┬Ā4.
The histopathological examination showed clear cells with round nuclei and abundant cytoplasm (H&E, ├Ś100).
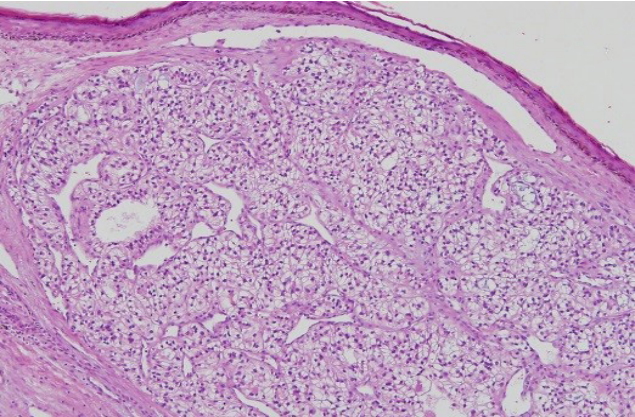
Fig.┬Ā5.
The split-thickness skin graft (STSG) coverage was planned to cover the newly formed raw surface due to rotational flap. The harvested STSG was held on the defect.
Table┬Ā1.
Summary regarding reported cases of renal cell carcinoma with metastasis to the scalp
| No. | Reporter | Sex/age (yr) | Treatment to primary tumor | Location of other metastasis | Treatment of metastasis | Time interval to skin metastasis (mo) | Survival after detection of metastasis (mo) |
|---|---|---|---|---|---|---|---|
| 1 | Errami et al. [1] | M/64 | Radical nephrectomy | Lung, tonsil, bone | Radiotherapy, tonsillectomy, chemotherapy | 24 | Not shown |
| 2 | de Paula et al. [5] | M/61 | Radical nephrectomy | Lung | Excision | 0 | 2 |
| 3 | Wahner-Roedler et al. [6] | F/72 | Radical nephrectomy | None | Radiotherapy | 0 | 15 |
| 4 | Huang et al. [7] | M/61 | Radical nephrectomy | Bone | Excision, radiotherapy | 0 | Not shown |
| 5 | Snow et al. [8] | F/69 | Radical nephrectomy | Bone | Excision with 5 mm margin, radiotherapy | 72 | Not shown |
| 6 | Dorairajan et al. [9] | M/55 | Radical nephrectomy | Brain | Chemotherapy | 10 | 4 |
| 7 | Dorairajan et al. [9] | M/55 | None | Liver, bone | None | 0 | 3 |
| 8 | Dorairajan et al. [9] | M/30 | None | Liver, brain, bone | Excision | 0 | 3 |
| 9 | Dorairajan et al. [9] | M/40 | None | Lung | Chemotherapy | 0 | 8 |
| 10 | Dorairajan et al. [9] | M/56 | Radical nephrectomy | Bone | None | 19 | 7 |
| 11 | Anzalone et al. [10] | M/52 | Chemotherapy | None | Excision with 3 mm margin | 30 | 24 |
| 12 | Pan et al. [11] | M/65 | Radical nephrectomy | None | Excision | 0 | Not shown |
| 13 | Livingston et al. [12] | M/45 | Radical nephrectomy | None | Excision | 0 | Not shown |
REFERENCES
1. Errami M, Margulis V, Huerta S. Renal cell carcinoma metastatic to the scalp. Rare Tumors 2016;8:139-41.

3. Amaadour L, Atreche L, Azegrar M, Benbrahim Z, Arifi S, Mellas N, et al. Cutaneous metastasis of renal cell carcinoma: a case report. J Cancer Ther 2017;8:603-7.

4. Byun SS, Hong SK, Lee S, Kook HR, Lee E, Kim HH, et al. The establishment of KORCC (KOrean Renal Cell Carcinoma) database. Investig Clin Urol 2016;57:50-7.



5. de Paula TA, da Silva PS, Berriel LG. Renal cell carcinoma with cutaneous metastasis: case report. J Bras Nefrol 2010;32:213-5.



6. Wahner-Roedler DL, Sebo TJ. Renal cell carcinoma: diagnosis based on metastatic manifestations. Mayo Clin Proc 1997;72:935-41.


7. Huang HC, Chang KP, Chen TM, Wu KF, Ueng SH. Renal cell carcinoma metastases in the head and neck. Chang Gung Med J 2006;29:59-65.
8. Snow S, Madjar D, Reizner G, MacK E, Bentz M. Renal cell carcinoma metastatic to the scalp: case report and review of the literature. Dermatol Surg 2001;27:192-4.


9. Dorairajan LN, Hemal AK, Aron M, Rajeev TP, Nair M, Seth A, et al. Cutaneous metastases in renal cell carcinoma. Urol Int 1999;63:164-7.


10. Anzalone CL, Cohen PR, Migden MR, Tannir NM. Mohs surgery in metastatic cancer: renal cell carcinoma solitary cutaneous metastasis and visceral tumor metastases to skin treated with microscopically controlled surgical excision. Int J Dermatol 2013;52:856-61.


11. Pan D, Niall O, Sharma H, Gya D. Isolated scalp nodule in patient with undiagnosed RCC. ScientificWorldJournal 2006;6:2430-2.




12. Livingston WD Jr, Becker DW Jr, Lentz CW 3rd. Solitary scalp metastasis as the presenting feature of a renal carcinoma. Br J Plast Surg 1977;30:319-20.


13. Escudier B, Eisen T, Porta C, Patard JJ, Khoo V, Algaba F, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23 Suppl 7:vii65-71.



14. Gong J, Maia MC, Dizman N, Govindarajan A, Pal SK. Metastasis in renal cell carcinoma: biology and implications for therapy. Asian J Urol 2016;3:286-92.



- TOOLS
-
METRICS

-
- 6 Crossref
- Scopus
- 5,135 View
- 178 Download
- Related articles in ACFS
-
Giant basal cell carcinoma of the left lateral neck2021 June;22(3)
Isolated temporalis muscle metastasis of renal cell carcinoma2021 February;22(1)
Aggressive cutaneous squamous cell carcinoma of the scalp2020 December;21(6)
A case of Merkel cell carcinoma of the head and neck2019 December;20(6)