A spindle cell squamous cell carcinoma on the cheek presenting with in-transit metastases and a satellite lesion
Article information
Abstract
Spindle cell squamous cell carcinoma (SpSCC) is a biphasic tumor composed of squamous cell epithelial and spindle cell mesenchymal components, both of which are malignant. Cutaneous SpSCC can cause diagnostic and therapeutic difficulties because of its rarity, heterogeneity, morphological similarity to other cutaneous spindle cell neoplasms, and uncertain pathogenesis and prognosis, particularly when the squamous cell carcinoma component is minimal or missing. Intransit metastasis and satellite lesion (satellitosis) constitute a spectrum of non-nodal regional metastases. Here the author reports the first known case of cutaneous SpSCC presenting with intransit metastases and a satellite lesion, which were exceptionally aggressive. A 77-year-old female patient presented with a 3× 3× 0.5 cm mass on her right cheek. Despite wide excision and postoperative radiation, the patient resulted in local recurrence and multiple distant metastases within 3 months. If many high-risk factors-particularly satellitosis and in-transit metastases are observed in a tumor with epithelial to mesenchymal transition, then further wide excision and adjuvant chemoradiation should be considered early in the treatment process. A multidisciplinary approach could be the key to cure the most aggressive malignancies of the skin, as in other organs.
INTRODUCTION
Spindle cell squamous cell carcinoma (SpSCC), also known as sarcomatoid squamous cell carcinoma (SCC), is a biphasic variant of SCC consisted of a mesenchymal component of sarcomatoid spindle cell proliferation and an epithelial component of SCC [1]. Cutaneous SpSCC is very rare and only 11 cases have been reported in Korea [2-4]. It can cause diagnostic and therapeutic problems particularly when the SCC component is minimal or absent because of ulceration or necrosis.
In-transit metastasis and satellite lesion (satellitosis) mean intralymphatic or, less frequently, angiotrophic tumor spread; these terms differ only in the distance of spread. When a regional metastasis is within 2 cm (an arbitrary cutoff) from the tumor, it is defined as satellitosis, whereas a regional metastasis beyond 2 cm is defined as in-transit metastasis [5-7]. The concept and clinical significance of in-transit metastasis and satellitosis have been established for melanoma, but they are very rare in other skin malignancies; overall only 59 in-transit metastases from SCCs have been reported [8-11].
Here the author reports an exceptionally aggressive cutaneous case of SpSCC, in which the patient showed a satellite lesion and recurrent in-transit metastases.
CASE REPORT
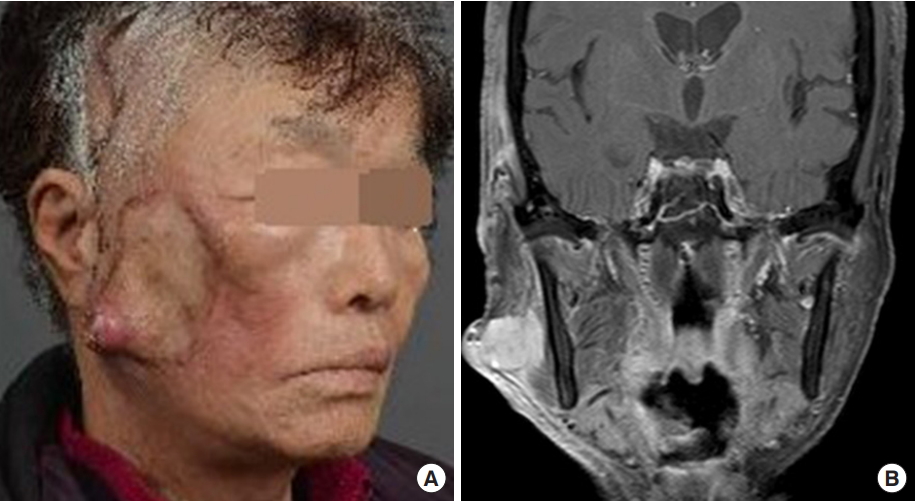
A 77-year-old woman presented with a 3× 3× 0.5 cm rubbery, hard, protruding mass on the right cheek. A small polyp developed 1 year ago and grew suddenly 1 month ago. It enlarged to 4× 5× 2 cm during 24 days of assessment and preparation for surgery. Computed tomography (CT) displayed a 2.5 cm irregular highly enhanced lesion. The preoperative work-up was negative on chest X-ray, neck CT and whole-body positron emission tomography/CT. Preoperative chemotherapy or radiation therapy was not administered due to the unwillingness of the elderly patient and consultation advice.
Intraoperatively, a new satellite lesion at 12 o’clock and three in-transit metastases at 3 to 6 o’clock were discovered. The satellite lesion was included within wide local excision of 2 cm safety margin and in-transit metastases were separately excised (Fig. 1). The zygomatic and buccal branches of facial nerve and parotid duct abutted the tumor and were sacrificed with partial en bloc excision of the parotid gland. Reconstruction was performed with a temporoparietal fascia transposition flap and full-thickness skin graft.
A 77-year-old female patient presented with a nontender, rubbery, hard, protruding mass measuring 3×3×0.5 cm with ulcers and a satellite lesion at 12 o’clock and three in-transit metastases at 3 to 6 o’clock on the right cheek.
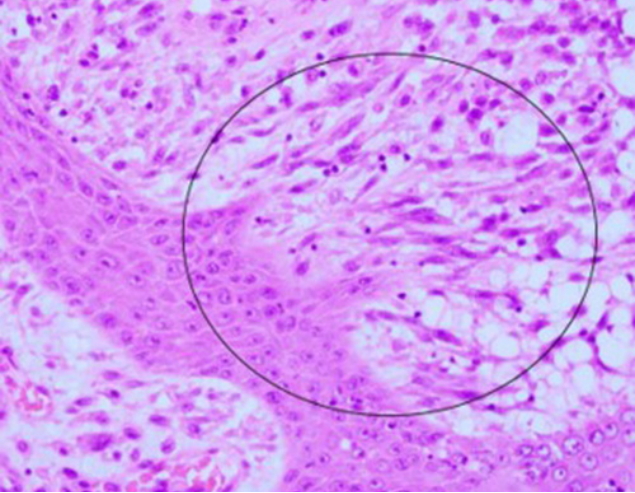
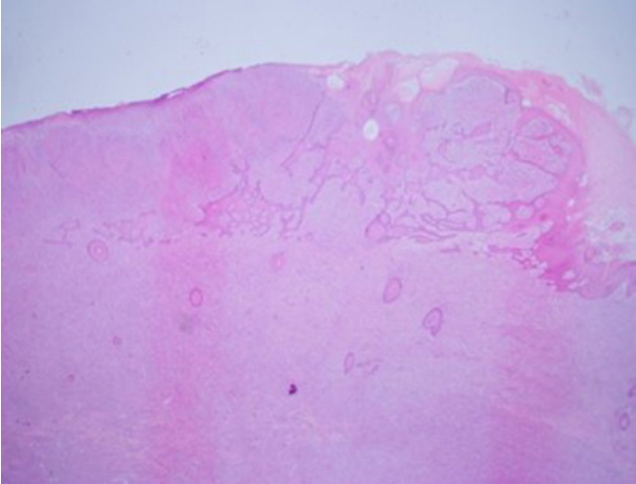
Microscopic examination revealed a well-differentiated SCC epithelial component and an undifferentiated sarcoma-like spindle cell mesenchymal component (Fig. 2). The mesenchymal component showed moderately pleomorphic features (spindle to round cytoplasms) with frequent mitoses. Some areas exhibited an obvious squamous to spindle cell transition, which excluded the possibility of collision of two separate tumors and suggested SpSCC or carcinosarcoma (Fig. 3). Immunohistochemical staining in sarcomatous area were positive for pancytokeratin, p40, and vimentin, but negative for smooth muscle actin, desmin, S100, CD68, and HMB-45 (Fig. 4). These findings excluded carcinosarcoma and resulted in the diagnosis of SpSCC with mesenchymal metaplasia (Table 1). Lymphovascular invasion was present without perineural invasion. The free lateral and deep margins were estimated as 18 mm and 1 mm respectively. Microscopic findings of in-transit metastases showed metastatic tumors with a clear resection margin.
Histopathological examination showing the biphasic malignant nature of tumor. The tumor had superficial epithelial and deep sarcomatous components. Squamous cell carcinoma of the epidermis infiltrated the upper dermis. The spindle tumor cells in the dermis and subcutis demonstrated atypical nuclei and frequent mitoses without any specific pattern of growth and cellular differentiation (H&E, ×10).
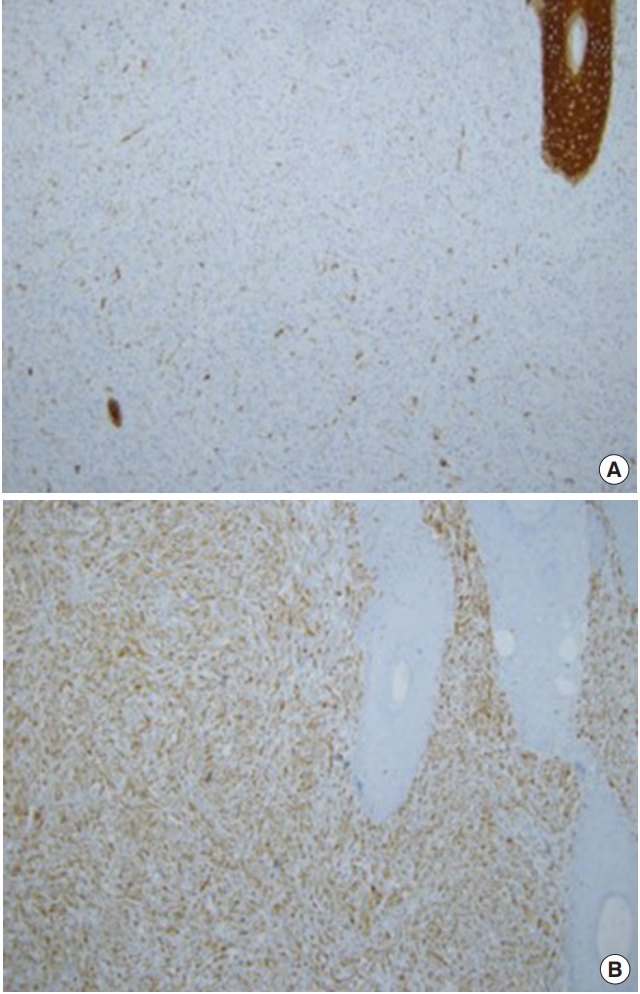
Photomicrographs of immunohistochemical staining. (A) Pancytokeratin staining. The squamous cell component in the upper right corner strongly expressed pancytokeratin, and a few tumor cells in the spindle component also expressed pancytokeratin (×100). (B) Vimentin staining. Tumor cells in the spindle cell component expressed vimentin strongly (×100).
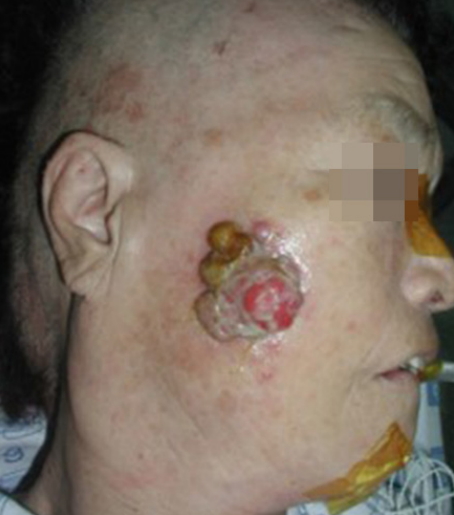
Starting on postoperative day 13, dark red serous fluid was aspirated repeatedly from a preauricular fluid collection without malignant cells on cytology. Seventeen days after the initial operation, four in-transit metastases were excised at 5 to 6 o’clock under local anesthesia (Fig. 5). Adjuvant radiation therapy was delayed until postoperative day 37 and then, after resolution of flap congestion and sialocele, radiation therapy was started to induce regression of a new 1-cm mass at 8 o’clock prior to a planned wide re-excision. Whether the recurrent mass was a remnant tumor or conduit spread along a nerve or a metastatic lymph node was unclear. The recurrent tumor shrunk initially but increased again during the 5-week radiation schedule (Fig. 6A). After 52 Gy of total radiation at the primary site, magnetic resonance imaging revealed a heterogenous mass measuring 3.8× 2 cm perforating the masseter deep to the mandible (Fig. 6B). The patient was transferred for re-excision and bone reconstruction. Multiple distant lung and adrenal metastases were discovered at the transferred hospital. The patient was lost to follow-up thereafter.
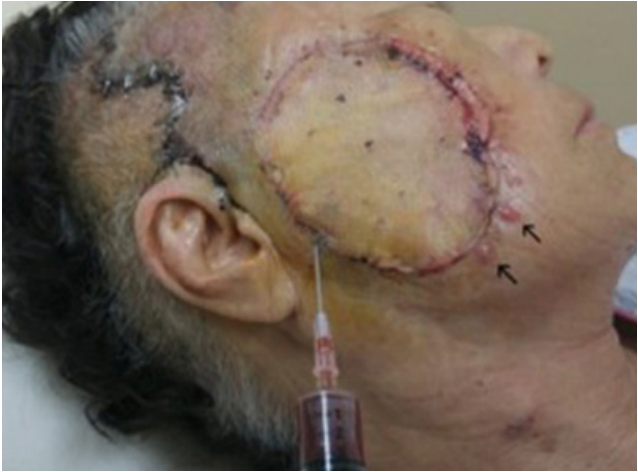
Recurrent episodes of in-transit metastases. At postoperative day 13, during aspiration of preauricular sialocele, four more intransit metastases were clearly seen at 5 to 6 o’clock (arrows).
DISCUSSION
Cutaneous SCC (cSCC) has a high cure rate, 5-year disease-free rates of 91% or higher [12]. However, cSCC has many variants and management should be individualized. Among these variants, some chimeric biphasic combinations exist such as SpSCC, basosquamous cell carcinoma and carcinosarcoma, which are mixtures of SCC with sarcomatoid spindle cells, basal cell carcinoma, and true mesenchymal sarcoma, respectively.
Three common pathogenetic hypotheses have been proposed to explain these hybrid tumors [13-15]: (1) collision of two synchronous unrelated tumors (collision theory); (2) combination of two divergent cell lines from a common pluripotential stem cell (combination or divergence theory); and (3) conversion from a less aggressive carcinoma to a more aggressive carcinoma or sarcoma by metaplastic transformation (conversion theory).
Most studies now consider them as metaplastic epithelial carcinomas (conversion theory) because most such diseases have a monoclonal origin with the presence of a transition zone (Fig. 3) and are thought to represent late-onset squamatization of basal cell carcinoma in basosquamous cell carcinoma or the epithelial to mesenchymal transition (EMT) in SpSCC and carcinosarcoma [13-15].
EMT is a ubiquitous developmental process during organogenesis in which embryonic epithelial cells lose their features and acquire a mesenchymal phenotype (spindle, epithelioid, and pleomorphic cells). EMT is not just a developmental phenomenon in embryos, but is also a pathogenetic mechanism in metaplastic tumors and a well-known facilitator of metastasis and angiogenesis [16]. During EMT, differentiated epithelial cells lose their characteristics, including cell adhesion and polarity, reorganize their cytoskeleton, and acquire spindle cell morphology and the ability to migrate.
The main differential diagnoses for cutaneous spindle cell malignancies are spindle cell melanoma, atypical fibroxanthoma, pleomorphic dermal sarcoma, angiosarcoma, leiomyosarcoma and carcinosarcoma (Table 1) [17,18]. In this case, the obvious presence of SCC precluded those entities except carcinosarcoma. The initial misdiagnosis of carcinosarcoma was later corrected to SpSCC by further immunohistochemical analyses of spindle cells. These immunohistochemical findings also indirectly support EMT as the pathogenetic mechanism.
Therapeutically, recent articles have suggested that the prognosis of cutaneous SpSCC is the same as that of ordinary cSCCs [2] in contrast to previous views of SpSCC as a more aggressive variant [1] although without a high-level of evidence. However, other grave prognostic factors such as a size greater than 4 cm, depth beyond the subcutaneous fat, abutment to nerve, and lymphovascular invasion placed this case as a high-risk lesion in National Comprehensive Cancer Network staging, T3 and stage III in the 8th edition of American Joint Committee on Cancer (AJCC-8) classification system, and T3 in the Brigham and Women’s Hospital staging system [19,20].
In particular, in-transit metastases and satellitosis should raise a red flag for staging and management. They are frequently reported in melanoma, and their behavior in melanoma has been well investigated. According to the AJCC-8 cancer staging, both have as poor prognosis as nodal metastasis and belong to the N “c” subcategory and stage IIIB or higher [6,7]. As in-transit metastasis and satellitosis indicate advanced stages, the choice of local treatment does not affect survival, which is dictated by development of distant metastases. Microscopically, in-transit lesions appear as sharply circumscribed nodules of tumor cells with a clear demarcation between normal tissue. Therefore, the standard local treatment for in-transit metastasis is complete excision rather than wide excision for primary melanoma. A satellite lesion is best included in wide excision.
In non-melanoma cutaneous malignancies, in-transit metastasis and satellitosis are very rare and therefore, the prognostic consequences are unclear. They are not mentioned in AJCC-8 staging system for head and neck nonmelanoma skin cancer [9,10,19]. However, the 5-year survival rate of 13% in the largest series [8] and as these phenomena represent regional metastasis and aggressiveness of the primary disease, adjuvant radiation therapy for locoregional control and chemotherapy for prevention of regional and distant metastasis should be considered. In addition to conventional chemotherapy for cSCC using cisplatin and 5-fluorouracil, some promising new therapies such as epidermal growth factor receptor inhibitors including cetuximab and panitumumab and immune checkpoint inhibitor pembrolizumab-have been approved by the Food and Drug Administration or are under clinical trials for noncutaneous head and neck SCC [20].
A clear deep margin was achieved grossly, and on intraoperative frozen and postoperative permanent analyses. However, the excision might have been insufficient because of its narrowness (1 mm), the use of bread-loaf sectioning instead of Mohs micrographic surgery, and the possibility of discontinuous tumor extension. However, the inevitable sacrifice of the facial nerve forced us to lean toward postoperative radiation therapy, rather th an further surgical resection at the operating table. Unfortunately, flap congestion and sialocele delayed radiation therapy until tumor recurrence, which was followed by distant metastasis shortly.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was approved by the Institutional Review Board of Chungbuk National University Hospital (IRB No. 2009-11-003) and performed in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained.
Patient consent
The patient provided written informed consent for the publication and the use of her images.