|
 |
- Search
| Arch Craniofac Surg > Volume 21(5); 2020 > Article |
|
Abstract
Background
Pilomatrixoma is a benign tumor that originates from the hair follicle matrix. It usually presents as a hard, slow growing, solitary mass that can be easily misdiagnosed as other skin masses. The aim of this study was to clinically analyze a case series of pilomatrixoma in pediatric patients from Korea.
Methods
A total of 165 pediatric patients from 2011 to 2018 with a histological diagnosis of pilomatrixoma were included. A retrospective review was performed using the electronic medical records, including patient demographics, number and location of the mass, clinical and imaging presentation, and postoperative outcomes.
Results
There were 61 male and 104 female patients with 152 solitary and 13 multiple pilomatrixomas. Among solitary pilomatrixomas, the lesion commonly occurred in the head and neck (84.2%), followed by upper limbs (11.2%), lower limbs (3.3%), and trunk (1.3%). The pilomatrixoma lesion presented as the following types based on our clinical classification: mass (56.02%), pigmentation (25.31%), mixed (12.65%), ulceration (4.82%), and keloid-like (1.2%). Ultrasonography showed a high positive predictive value (95.56%). There were no specific complications observed except for two cases of recurrence.
Pilomatrixoma, also known as pilomatricoma or calcifying epithelioma of Malherbe, was first described by Malherbe and Chenantais in 1880 [1]. In 1961, Forbis and Helwig [2] proposed the term âpilomatrixomaâ instead of âcalcifying epithelioma,â because studies showed definite similarities in both tumor and hair, thus elucidating the hair follicle matrix as the point of origin. Pilomatrixoma is a rare benign tumor with an incidence of approximately 1% among benign skin lesions [3].
Previous studies revealed pilomatrixoma presentation in young patients aged less than 20 years with a female predominance. The head, neck, and extremities are the main regions in which the tumor occurred. Pilomatrixoma typically presents as a solitary lesion, although some patients still present multiple lesions [4].
Clinically, pilomatrixoma commonly presents as a hard, freely mobile, and slow-growing mass. Patients are usually asymptomatic, but in some cases, patients complain of pain or discharge from the lesions [5,6]. Pilomatrixoma is located in the deep dermis or in the subcutaneous layer, presenting as a subcutaneous mass. Occasionally, clearly demarcated vessels or skin ulcerations can even be seen as the overlying skin attenuates [6,7]. The varied clinical appearance of pilomatrixoma is associated with a diagnostic flaw, wherein it is easily misdiagnosed as other skin masses, such as epidermal cyst or dermoid cyst, resulting in a low percentage of correct preoperative diagnosis 16% [7,8]. In our opinion, it is necessary to classify the types of pilomatrixoma because of its varied presentations, thereby enabling easier diagnosis. Therefore, we investigated the clinical data of pediatric patients with pilomatrixoma, especially focusing on the clinical features and imaging findings. There have been few records of case reports on pilomatrixoma and a single study of pilomatrixoma in adults [9] to date; thus, this is the first large-scale clinical study of pilomatrixoma in pediatric patients from Korea.
All pediatric patients from 2011 to 2018 who were surgically treated for mainly subcutaneous mass at the department of plastic and reconstructive surgery were evaluated. We reviewed the electronic medical records of the patients, including their clinical and pathological results. A total of 165 pediatric patients with a histological diagnosis of pilomatrixoma were identified for this retrospective analysis. For this study, ethical approval was obtained from the Institutional Review Board of Seoul National University Hospital (IRB No. 1902-102-1011). The clinical data included the patientsâ sex, age at operation, location of the mass, solitary or multiple lesions. The presentations were classified accordingly into five different types based on clinical characterization. Imaging results were reviewed to analyze the characteristics of pilomatrixoma and determine the diagnostic accuracy. Investigation of postoperative outcomes included complications or recurrence.
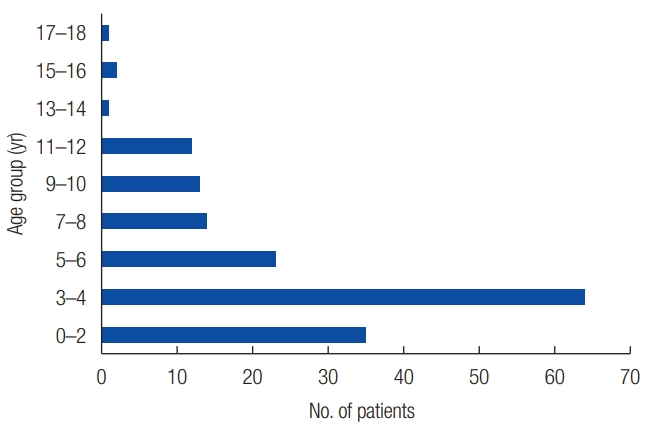
Among the 165 patients, 61 (36.97%) were male and 104 (63.03%) were female; there was an observed female predominance with a male-to-female ratio of 1:1.70. A total of 152 patients (92.12%) had a solitary pilomatrixoma (SP) wherein 53 were males and 99 were females. The remaining 13 patients (7.88%) had multiple pilomatrixomas (MPs) wherein eight were males and five were females (Table 1). In all of these cases, the patientsâ age at the time of operation ranged from 4 months to 18 years, with a median age of 4 years. One hundred and forty-nine cases (93.3%) occurred before 10 years of age (Fig. 1).
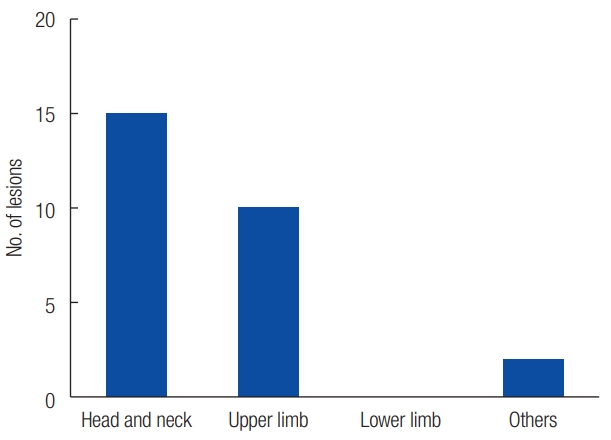
In SP, the most commonly affected region was the head and neck (n=128), followed by upper limbs (n=17), lower limbs (n=5), and back (n=2) (Fig. 2A). In the head region, mostly occurred in the cheek (n=43), followed by auricular site (n=35), eyebrow (n=16), eyelid (n=10), temple (n=8), forehead (n=3), glabella (n=1), and the nasolabial fold (n=1) (Fig. 2B). Of the 13 MP cases, 12 presented with two lesions, and one presented three lesions. The most commonly affected region was the head and neck (n=15), followed by upper limbs (n=10) and back (n=2) (Fig. 3).
Among the 152 lesions in SP and 27 lesions in MP, medical documentations were obtained preoperatively in 166 lesions. These photos were evaluated by three plastic surgeons (JLH, HY, and BJK) based on the characteristic appearance of the pilomatrixoma. These lesions were classified into five clinical types, which included the mass, pigmentation, mixed type, ulceration, and keloid-like types. The mass type was the predominant presentation in 93 lesions characterized as a skin protrusion. Additionally, among the mass type lesions, two lesions showed a conspicuous âtent signâ with multiple facets and angles (Fig. 4A and B). There were 42 lesions classified as pigmentation type, which is characterized by an obvious skin color change with vessels or melanin-like pigmentation (Fig. 4C and D). There were 21 lesions classified as mixed type that showed characteristics of both mass and pigmentation types (Fig. 4E). There were eight lesions classified as ulceration type, which is characterized by a calcification discharge shown through defects in the skin (Fig. 4F). Finally, there were two lesions classified as keloid-like type, which is characterized by an overgrown similar to a keloid scar (Table 1, Fig. 4G).
Preoperative diagnostic imaging was performed to identify pilomatrixoma. The imaging modalities included ultrasonography (US), computed tomography (CT), or magnetic resonance imaging (MRI). There were 90 patients who underwent US with a positive predictive value of 95.56% (86/90). According to the US images, the mean size of pilomatrixomas was 9.47Âą11.51 mm. Additionally, one patient underwent CT at a local hospital before visiting our institution and the result stated that the lesion was a pilomatrixoma. Furthermore, two patients underwent MRI in order to rule out vascular lesions in one case, and to confirm the anatomy of the facial nerve, to avoid neural injury as it was located close the lesion in the other case.
Surgical removal was performed in all patients under general anesthesia. Preoperative laboratory examinations, including blood tests, urine examination, chest radiography, and electrocardiogram, were performed in order to assess the general indications for anesthesia. There were no postoperative complications such as infection, hematoma, or wound dehiscence. The average follow-up period was 6.12Âą10.24 months. Two patients (1.2%) had recurrent pilomatrixoma that was located in the eyelid and preauricular area, with an average of 15.5 months recurrence time postoperatively. There were no cases of malignant transformation or metastatic malignant pilomatrixoma.
Pilomatrixoma, arising from the hair follicle matrix, is generally a benign tumor occurring in the deep dermis or subcutaneous layer. It usually presents as a solitary lesion, although it presents as multiple lesions in approximately 2% to 9% of cases [8]. In our study, 7.88% of cases presented with MP. The cause of pilomatrixoma remains unclear, previous studies have suggested that the development of pilomatrixoma is related to β-catenin mutation [10]. In addition, MP is reported to be associated with familial inheritance or general syndromic conditions, such as myotonic dystrophy, Gardner syndrome, and Turnerâs syndrome [11].
Epidemiologically, pilomatrixoma can occur at any age. Previous studies showed that there are two age peaks in which pilomatrixoma occurs intensively: first peak is in young patients aged between 0 and 20 years, whereas the second is in older patients aged between 40 and 60 years [12]. A retrospective study of 2,189 cases demonstrated that more than half of pilomatrixomas occurred in pediatric patients aged <20 years [8]. Moreover, among pediatric patients, the majority of cases occurred before 10 years of age [8,13]. In this study, 149 cases (93.3%) occurred before 10 years of age which is consistent with that reported in previous studies. The four cases of pilomatrixoma collected in adults during the same period were excluded from this study.
Based on previous retrospective studies, both SP and MP presentation had a female predominance [8,13]. In addition, the most common area of pilomatrixoma occurrence was in the head and neck, mainly in the cheek or the palpebral area, followed by the upper extremities, trunk, and lower extremities [4]. Similarly, in this study, female predominance (63.03%) was also observed with a common occurrence in the head and neck region.
Moreover, the clinical manifestations of pilomatrixomas are diverse. Generally, pilomatrixoma is a hard, freely mobile, slow-growing mass covered by skin. Patients are asymptomatic, although in some cases pilomatrixoma may present with symptoms, such as pain, pruritus or discharge in lesions [5-7], which makes the diagnosis more difficult. In our study, five clinical types of pilomatrixoma presentation were established according to the characteristic appearance of the lesions. The mass type was the most common (56.02%), with a firm calcified nodule protruding from the skin. In some cases, the tumor showed multiple facets and angles referred to as âtent signâ [14], which is a helpful feature for the diagnosis of pilomatrixoma. The pigmentation type was the second most common presentation of pilomatrixoma (25.31%). Bowers and Millard [15] showed that pilomatrixoma could be covered by small vessels that made the skin red or blue in color. In addition, Ishida and Okabe [16] showed that melanin pigment and melanocytes existed in the pilomatrixoma, identified as pigmented pilomatrixoma. There were 21 lesions (12.65%) that presented as protuberant nodules with discoloration of the skin, and these were classified as the mixed type. Moreover, once a pilomatrixoma forms in the superficial papillary or mid-dermis, the tumor may perforate from the skin through a process of transdermal elimination, leading to ulceration [4,17]. This characteristic is rare with only 4.28% cases presenting with perforating pilomatrixomas, and these were classified as the ulceration type. There was a special type defined as the keloid-like type in two lesions. Compared with the mass type, the keloid-like type lesions were characterized by excessive growth beyond the original area of the lesion; thus, it was easily misdiagnosed as a keloid or hypertrophic scar. The varieties of pilomatrixoma clinical features contribute to the increased tendency for misdiagnosis for other skin lesions. Differential diagnosis of epidermoid or dermoid cyst, calcified hematoma, and foreign body reaction are necessary considerations.
In order to improve the diagnostic rate of pilomatrixoma, preoperative imaging including US, CT, or MRI is recommended. Compared with CT and MRI, US is considered a non-anesthetic, noninvasive, low-priced, quick, and acceptable approach for children with high accuracy of more than 80% [18]. In this study, US had a positive predictive value of 95.56%. Generally, on US, the pilomatrixoma is described as an oval, well-defined, heterogeneous, hyperechoic subcutaneous mass with or without posterior shadowing [18,19] (Fig. 5).
Complete surgical resection is the treatment of choice for pilomatrixomas. The tumor occurs predominantly in the head and neck region; thus, indirect incisions are often used for cosmetic reasons. Take, for example, a hairline incision adapted to approach the mass in the forehead or the temple for cosmesis. These surgical procedures should be carefully approached to avoid injury to the facial nerve or sensory nerve branches of the face. After tumor removal in the giant pilomatrixoma, the defective skin should be reconstructed with a flap [20,21].
Although recurrent pilomatrixoma is rare, approximately 2% of cases still recur [8]. Some case reports showed that the postoperative recurrence time ranged from 1 year to decades [22,23]. In our study, the average recurrence time in two cases (1.2%) was 15.5 months, postoperatively. The cause of recurrence is generally due to incomplete resection, especially when the tumor occurs in a sensitive location; thus, sufficient resection margins are recommended to reduce the recurrence rate [23].
Malignant transformation of the pilomatrixoma is very rare. Previous case reports and case series indicated that malignant transformation occurred only in adult patients [24,25]. However, Otero et al. [26] has reported the first pediatric case of a malignant pilomatrixoma transformation from a previous operation site; thus targeted clinical evaluation should be performed to rule out the risk of malignant changes in recurrent pilomatrixomas.
In conclusion, pilomatrixoma commonly presents as a solitary mass lesion on the face. Although the clinical appearances of pilomatrixoma vary, making misdiagnosis common, the established clinical classifications of pilomatrixoma presentations may help in the identification when compared with other skin tumors. US is also a helpful imaging tool for the diagnosis of pilomatrixoma. Complete surgical excision is the treatment of choice with a low recurrence rate.
Notes
Fig. 2.
Affected regions in solitary pilomatrixoma. (A) Distribution of pilomatrixoma on the whole body. (B) Distribution of pilomatrixoma on the head.
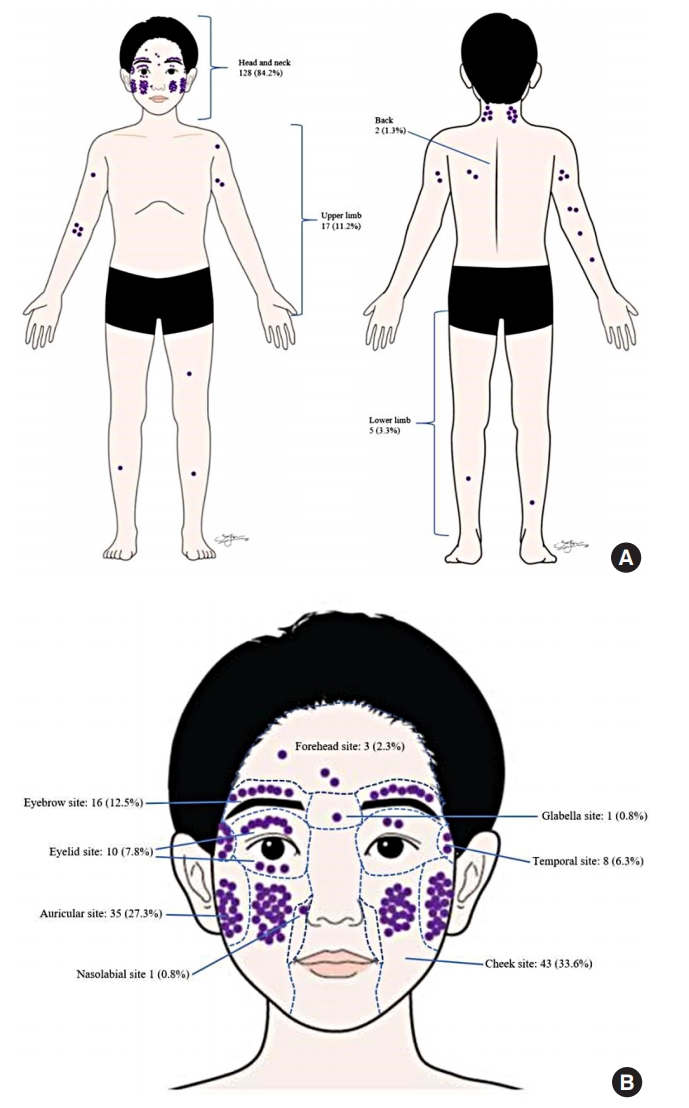
Fig. 4.
Clinical documentation of the different types of pilomatrixoma. (A) Mass type. (B) Typical mass type with the âtent sign.â (C) Pigmentation type with clearly demarcated vessels. (D) Pigmentation type with melanin-like pigmentation. (E) Mixed type. (F) Ulceration type. (G) Keloid-like type.
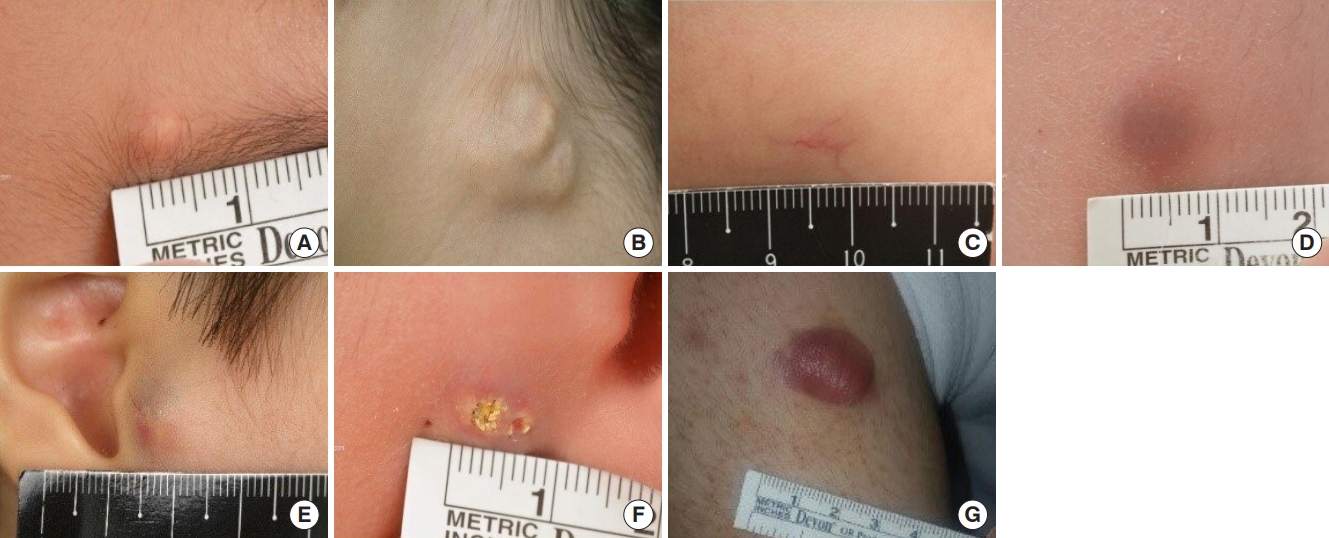
Fig. 5.
The ultrasound image shows a well-defined heterogeneous hyperechoic nodule with low echoic rim and internal calcifications in the subcutaneous layer of the right calf. Note the increased echogenicity in the surrounding subcutaneous fat layer and conspicuous acoustic shadowing.

Table 1.
Demographics of patients and clinical classifications of pilomatrixoma
REFERENCES
1. Malherbe A, Chenantais J. Notes on calcifying epitheliomas of sebaceous glands. Prog Med 1880;8:826-8.
4. Abdeldayem M, Mekhail P, Farag M, Shehata G, Al Sheikh M, Izzidien A, et al. Patient profile and outcome of pilomatrixoma in district general hospital in United Kingdom. J Cutan Aesthet Surg 2013;6:107-10.



5. Koh IS, Cho HJ, Kim JW. Rapidly growing giant pilomatricoma in the right parotid region of a pregnant woman. Arch Craniofac Surg 2020;21:176-9.




6. DeRosa DC, Lin-Hurtubise K. Pilomatricoma: an unusual dermatologic neoplasm. Hawaii J Med Public Health 2012;71:282-6.


7. Pant I, Joshi SC, Kaur G, Kumar G. Pilomatricoma as a diagnostic pitfall in clinical practice: report of two cases and review of literature. Indian J Dermatol 2010;55:390-2.



8. Jones CD, Ho W, Robertson BF, Gunn E, Morley S. Pilomatrixoma: a comprehensive review of the literature. Am J Dermatopathol 2018;40:631-41.


9. Han G, Kim AR, Song HJ, Oh CH, Jeon J. Updated view on epidemiology and clinical aspects of pilomatricoma in adults. Int J Dermatol 2017;56:1032-6.


10. Moreno-Bueno G, Gamallo C, Perez-Gallego L, Contreras F, Palacios J. beta-catenin expression in pilomatrixomas. Relationship with beta-catenin gene mutations and comparison with beta-catenin expression in normal hair follicles. Br J Dermatol 2001;145:576-81.


11. Mesa-Alvarez L, Batalla A, Iglesias-Puzas A, Alvarez C, Florez A. Multiple pilomatricomas: a retrospective study and literature review. Am J Dermatopathol 2019;41:293-5.


12. Lan MY, Lan MC, Ho CY, Li WY, Lin CZ. Pilomatricoma of the head and neck: a retrospective review of 179 cases. Arch Otolaryngol Head Neck Surg 2003;129:1327-30.


13. Pirouzmanesh A, Reinisch JF, Gonzalez-Gomez I, Smith EM, Meara JG. Pilomatrixoma: a review of 346 cases. Plast Reconstr Surg 2003;112:1784-9.


15. Bowers PW, Millard LG. Clinical presentation of calcifying epitheliomas of Malherbe. Br J Dermatol 1981;105(Supple 19):31.
16. Ishida M, Okabe H. Pigmented pilomatricoma: an underrecognized variant. Int J Clin Exp Pathol 2013;6:1890-3.


17. Ohnishi T, Nakamura Y, Watanabe S. Perforating pilomatricoma in a process of total elimination. J Am Acad Dermatol 2003;49(2 Suppl Case Reports):S146-7.

18. Hwang JY, Lee SW, Lee SM. The common ultrasonographic features of pilomatricoma. J Ultrasound Med 2005;24:1397-402.


19. Choo HJ, Lee SJ, Lee YH, Lee JH, Oh M, Kim MH, et al. Pilomatricomas: the diagnostic value of ultrasound. Skeletal Radiol 2010;39:243-50.



20. Jimenez-Puya R, Vazquez-Vayo C, Rodriguez-Bujaldon A, Gomez-Garcia F, Moreno-Gimenez JC. Proliferating pilomatricoma treated using a bilateral advancement flap. Actas Dermosifiliogr 2009;100:730-2.


21. Kim YS, Na YC, Huh WH, Kim JM. Malignant pilomatricoma of the cheek in an infant. Arch Craniofac Surg 2018;19:283-6.




22. Hmar V, Thokchom N, Kshetrimayum S. Recurrent pilomatricoma of the thigh: an unusual site of presentation. Indian J Pediatr Dermatol 2018;19:65-7.

23. Levy J, Ilsar M, Deckel Y, Maly A, Anteby I, Peâer J. Eyelid pilomatrixoma: a description of 16 cases and a review of the literature. Surv Ophthalmol 2008;53:526-35.


24. Liu JF, Li B, Fan ZX, Jiao T, Li C, Qin S, et al. Pilomatrix carcinoma on the left side of the parotid region: a case report and review of the literature. Oncol Lett 2015;10:313-6.



- TOOLS
-
METRICS

-
- 20 Crossref
- Scopus
- 6,065 View
- 149 Download
- Related articles in ACFS