Aggressive cutaneous squamous cell carcinoma of the scalp
Article information
Abstract
Cutaneous squamous cell carcinoma (cSCC) is the second most common nonmelanoma skin cancer, and its incidence is increasing globally. In Korea, there were 12,516 diagnosed cases of cSCC between 1999 and 2014. Surgical treatment, for which several options are available, is the standard of care for cSCC and securing a sufficient surgical resection margin is always important. cSCC of the scalp sometimes exhibits unusually aggressive behavior. In this article, we report a case of cSCC of the scalp with invasion into the skull and dura mater.
INTRODUCTION
Cutaneous squamous cell carcinoma (cSCC) is the second most common nonmelanoma skin cancer, and its incidence is steadily increasing [1]. Although the majority of cSCCs are successfully eradicated by surgical excision, advanced cSCC poses a significant risk in terms of morbidity, impact on quality of life, and risk of death. Therefore, proper management of advanced cSCC is of the utmost importance since local invasion, delayed diagnosis, and metastasis contribute to increased costs and morbidity [2].
The incidence of tumors on the scalp is also increasing and cSCC is the second most prevalent malignant. Partly because cSCC of the scalp are often diagnosed at an advanced stage, this location may be considered an independent risk factor for invasiveness, increased risk of recurrence and therefore a more unfavorable prognosis [1].
In this article, we report a case of aggressive cSCC of the scalp with invasion into the skull and dura mater and emphasize the importance of performing sufficient resection to ensure an adequate safety margin and utilizing a multidisciplinary approach.
CASE REPORT
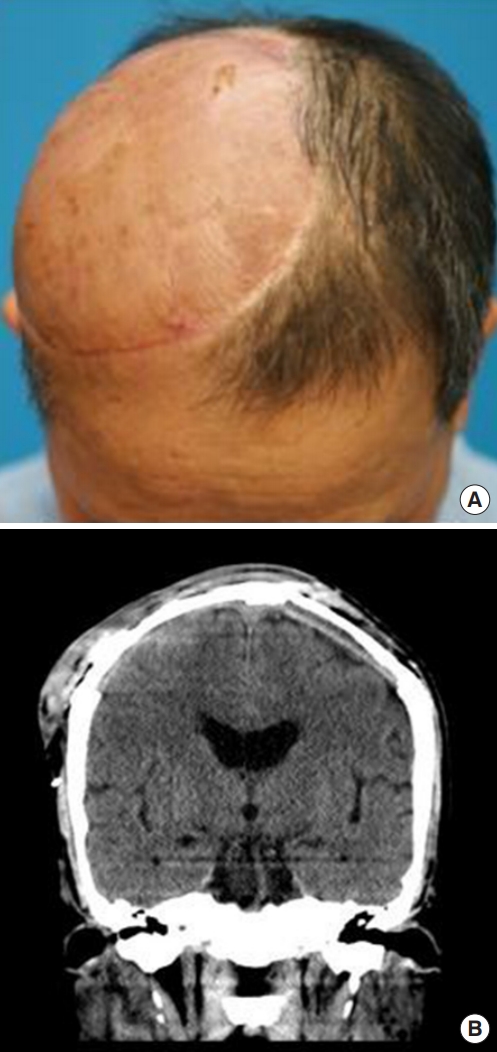
A 60-year-old man presented to the department of dermatology complaining of an ulcerative lesion on the scalp. The patient had a scalp burn 50 years before and had a history of recurrent, unhealed ulcerative lesions. On physical examination, a 6×4 cm erosive verrucous plaque was observed in the right frontoparietal region with ulceration and pain upon palpation. Preoperative computed tomography (CT) showed a bone-destructive mass in the frontoparietal region and did not reveal any neck lymph node metastasis. Based on a punch biopsy, well-differentiated squamous cell carcinoma was diagnosed. The department of dermatology planned surgical excision with adjuvant radiotherapy (RT). A physician performed wide local excision and a full-thickness skin graft. After surgical excision, brain magnetic resonance imaging (MRI) and positron emission tomography (PET) scanning were conducted before RT. However, the brain MRI and PET scans showed residual malignant tissue with dural invasion (Fig. 1). Thus, for curative secondary surgery, the patient was transferred to the neurosurgery department (Fig. 2).
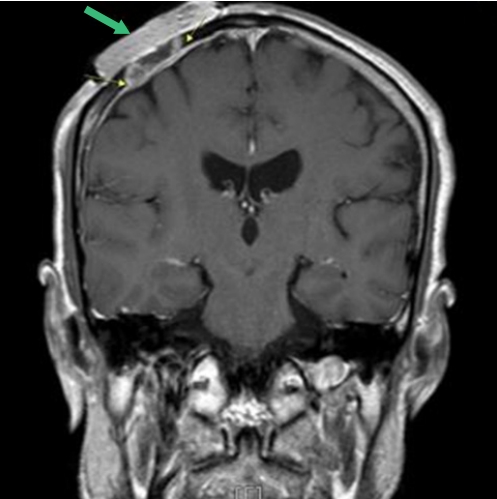
Diffuse pachymeningeal enhancement, deep to previous skin graft area, indicated dural invasion (green arrow indicate dressing material).
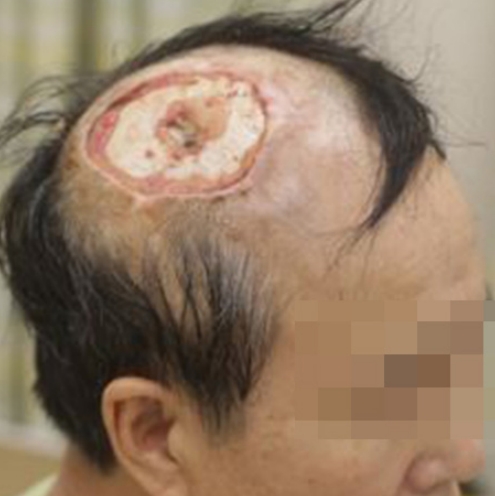
When the patient was referred for curative secondary surgery, wide scalp defect (about 8 × 7 cm) with bone exposure was observed.
The treatment plan was designed using a multidisciplinary approach, with collaboration of neurosurgery, hemato-oncology, radiation oncology, radiology, and nuclear medicine teams. Physicians in our department and in the neurosurgery department decided on the following surgical plan: (1) wide excision with bone and dura resection; (2) duraplasty using artificial dura material and cranioplasty with autologous skull splitting; and (3) reconstruction using a latissimus dorsi muscle free flap and split-thickness skin graft.
The surgical excision margin was 2 cm. Frozen biopsy revealed no evidence of residual tumor cells. After wide excision, the defect measured approximately 17×15 cm. We elevated a latissimus dorsi muscle free flap. The thoracodorsal artery was anastomosed end-to-end with the superficial temporal artery, and the thoracodorsal vein was anastomosed end-to-end with the superficial temporal vein. For coverage of the muscle flap, thigh skin was harvested and a meshed graft was done (Figs. 3, 4).
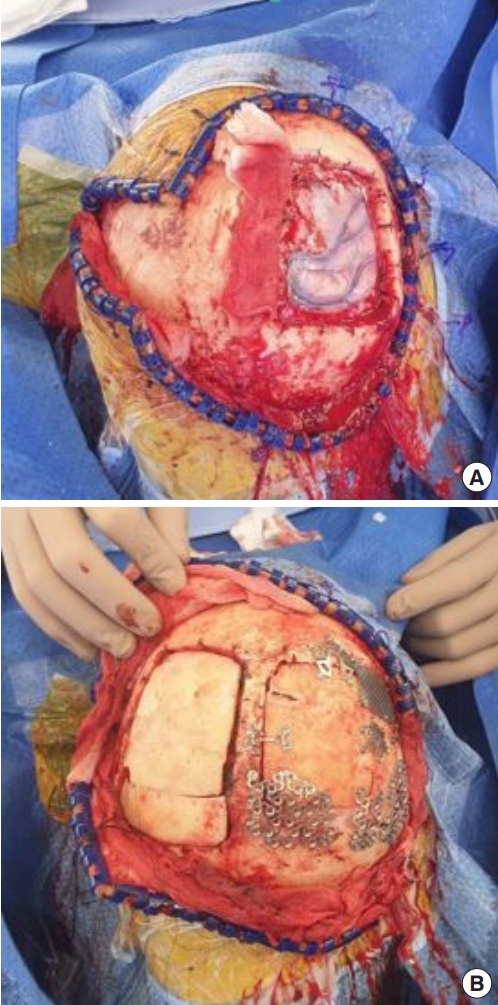
Neurosurgery department did wide excision with duraplasty and cranioplasty. (A) Dura defect about 8 × 8 cm was covered with artificial dura material. (B) Bone defect about 10 × 10 cm was reconstructed with bihalved calvarial bone graft.
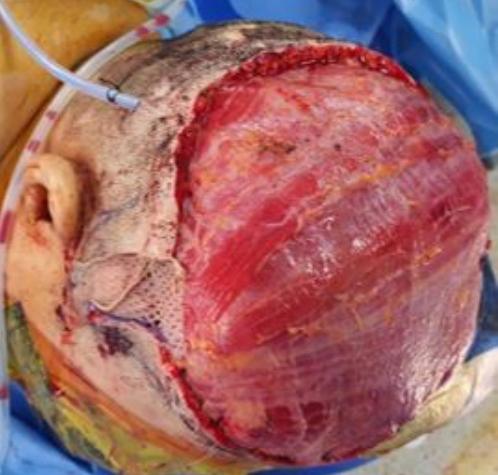
Defect measuring approximately 15 × 17 cm remained on scalp and latissimus dorsi muscle free flap was elevated to reconstruct the defect area.
A histopathologic examination revealed squamous cell carcinoma cells infiltrating dermis layer (Fig. 5). The pathology findings were the same as reported in the first biopsy.
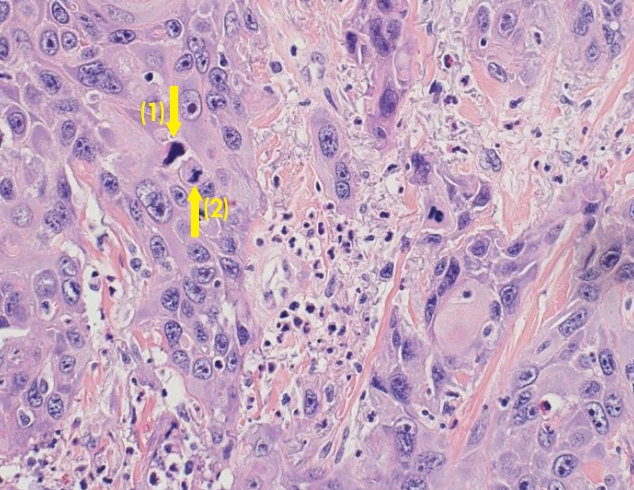
High-power view shows squamous cell carcinoma cells infiltrating dermis (H&E, × 400). Marked anisonucleosis, large nucleoli, coarse chromatin, and occasional bizarre nuclei (1) can be seen. Numerous mitotic figures including atypical mitosis (2) are also noted.
The flap survived without any complications and cerebrospinal fluid leakage and neurologic deficit were not observed. The patient was discharged after 1 month and postoperative RT was planned.
At 3 months of postoperative follow-up, there was no evidence of systemic relapse on the CT scan. An examination of the surgical scar did not reveal any local recurrence (Fig. 6).
DISCUSSION
cSCC is the second most common skin cancer and the second most common form of carcinoma after basal cell carcinoma. The incidence of cSCC is increasing throughout the world. In the United States, the lifetime risk for development of cSCC is estimated to be 9% to 14% for men and 4% to 9% for women [3]. In Korea, there were 12,516 diagnosed cases of cSCC between 1999 and 2014. Its prevalence has also steadily increased from 0.59% to 1.11% in women and from 0.95% to 1.28% in men during 1999–2002 and 2011–2014 [4].
The incidence of tumors on the scalp is increasing compared to those occurring elsewhere on the skin. Only approximately 1% to 2% of all scalp tumors are malignant, they comprise up to 13% of all malignant cutaneous neoplasms and cSCC is the second most prevalent malignant [5]. Chiu et al. [6] examined 398 Taiwanese patients with malignant scalp tumors and found that basal cell carcinoma (41.2%) was the most common malignant scalp tumor, followed by squamous cell carcinoma (16.6%).
Surgical treatment remains the standard of care for cSCC, and several surgical treatment options exist. The most widely used surgical excision modalities are standard excision with postoperative margin assessment, wide local excision, and Mohs micrographic surgery. There is no single surgical treatment of choice, but securing a sufficient surgical resection margin is of the utmost importance.
The National Comprehensive Cancer Network (NCCN) guidelines suggest the margin selection for cSCC as low- and high-risk, and these recommendations were substantiated by the findings of Brodland and Zitelli [7]. For tumors size larger than 6 mm in high-risk locations, larger than 10 mm in moderate risk locations, or tumors penetrating to the level of subcutaneous fat on biopsy, the NCCN advises surgical excision with peripheral margins greater than 6 mm. High-risk lesions are those that are ill-defined and affect the genitalia, mucosal surfaces, scalp, and neck [8]. In our case, considering the presence of Marjolin’s ulcer and the high-risk status of the lesion, the surgical excision margin was 2 cm and the deep margin was negative.
Depth of invasion is a significant prognostic indicator of cSCC of the scalp. In a retrospective study, dural involvement reduced 3-year survival from 83% to 22% [9]. Advanced scalp cancer may often be considered inoperable when the dura or brain parenchyma is involved. So handling these problems in the head and neck region often requires close cooperation among specialists. Therefore, a multidisciplinary approach including a neurosurgeon and a plastic surgeon is often necessary to ensure safe tumor extirpation and adequate reconstruction.
There are several surgical methods to reconstruct scalp such as skin grafts, local tissue transfer and free flaps and several factors need to be considered when selecting ideal methods. The size of the defect, anatomic involvement, contour restoration, hairline maintenance, and return of soft-tissue bulk must all weigh in during the decision making process [10]. The new tissue must also be able to withstand the shear forces that may be applied to it in the future, heal in a timely fashion to allow adjuvant treatments [11]. If postoperative radiation therapy is necessary, additional considerations arise. Skin grafting in such cases provides insufficient durability, and local tissue transfer has inferior vascular supply when compared with free flaps. Free flaps supply healthy tissue with their concomitant blood supply and can withstand radiation. Multiple free flaps have been described, including latissimus dorsi, anterolateral thigh, radial forearm and rectus abdominus. Overall, the free latissimus dorsi muscle flap has become the preferred free flap for scalp reconstruction due to its large surface area, and long vascular pedicle. In addition, it can be elevated as a pure muscle or musculocutaneous flap [12].
The incidence of cSCC is increasing and some cSCCs of the scalp exhibit unusually aggressive behavior. In this case report, we highlight the importance of performing complete resection with a sufficient safety margin for local disease control and utilizing a multidisciplinary approach.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was approved by the Institutional Review Board of Kangbuk Samsung Hospital (IRB No. KBSMC 2020-06-043) and performed in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained.
Patient consent
The patient provided written informed consent for the publication and the use of his images.