Effects of perioperative radiation treatment on stricture and fistula formation in hypopharyngeal reconstruction: a meta-analysis
Article information
Abstract
Hypopharyngeal reconstruction is a surgically challenging procedure, and postoperative management is important due to a high rate of complications following surgery. In particular, stricture and fistula formation is the most common long-term postoperative complication. Through systematic review and meta-analysis of 21 studies, a significant radiation effect of stricture and fistula formation was found in patients who underwent hypopharyngeal reconstruction. The perioperative radiation must be seen as a critical factor for stricture and fistula formation in hypopharyngeal reconstruction.
INTRODUCTION
Reconstruction of the hypopharynx due to extensive oncologic surgery is one of the most difficult and challenging procedures for a surgeon. According to advancements in microsurgical techniques, free tissue transfer for reconstruction of the hypopharyngeal defect has become the primary choice of treatment [1,2]. Although free flap surgery is a single-stage repair procedure and could minimize any postoperative complications, morbidity after hypopharyngeal reconstruction remains high [3]. In particular, stricture and fistula are the most common and threatening complications [4,5]. These local complications can lead to salivary leakage, swallowing problems, and even fatal carotid artery rupture.
For minimizing the occurrence of these local complications such as stricture and fistula, verifying the predisposing factors affecting the postoperative results has been an important issue in previous studies. Considering the predisposing treatment factors for hypopharyngeal defect is important because the clinician can predict the postoperative results and try to prevent or minimize these complications. Among these treatment options, preoperative and postoperative radiation treatments have been often implicated in stricture and fistula formation [6,7]. However, the radiation factors for such postoperative complications are still controversial, and no definite studies are presently available. Therefore, the establishment of an association between radiation treatment and postoperative complications, such as stricture and fistula formation, would be beneficial for oncologic patients of the hypopharynx.
Through a review of relevant studies, this study aimed to explore the stricture and fistula formation rate after preoperative or postoperative radiation treatment in patients who underwent hypopharyngeal reconstruction and to determine the degree of association between radiation and these complication rates.
IDENTIFICATION OF RELEVANT STUDIES
A search for eligible articles using the PubMed, Embase, and Cochrane Library databases for all studies published prior to January 2020 was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist [8]. The purpose of the literature search was to verify the effect of perioperative radiation treatment in patients who underwent hypopharyngeal reconstruction. The search terms included “hypopharynx,” “pharyngoesophageal,” “pharyngolaryngectomy,” “pharyngectomy,” and “pharyngeal.” Only human studies were included, and relevant articles were examined for references to additional eligible studies.
The inclusion criteria were as follows: (1) a full-length article that provided sufficient data to evaluate the treatment results of preoperative or postoperative radiation treatment in patients who underwent hypopharyngeal reconstruction; (2) a brief description of the location of the operation and whether radiation treatment was used; (3) prospective or retrospective trials; (4) a brief explanation of outcome variables (complication rate such as stricture or fistula formation). Studies were excluded if they involved incomplete or interim data; were written in languages other than English; did not contain information regarding the treatment results; were a case report, review article, letter, or communication; or the authors were overlapping.
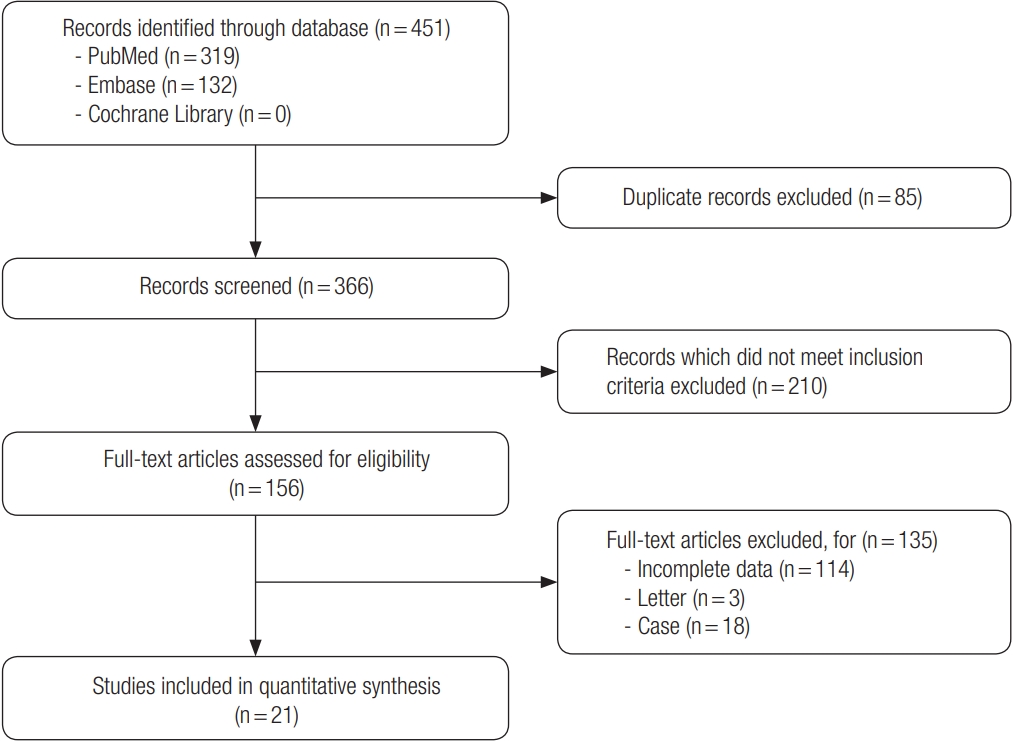
Fig. 1 shows the flow diagram of how the eligible studies were investigated. The database searches identified 451 publications that potentially met the study criteria. Duplicate 85 records from 451 studies were excluded. The screening process, consisting of a review of titles and abstracts, excluded 210 studies that did not meet the inclusion criteria. A total of 156 full-text articles were reviewed for eligibility. The reasons for study exclusion during the final review were as follows: incomplete data (n= 114), letter (n= 3), and case report (n= 18). The remaining 21 non-randomized studies were included in the final analysis [1,5,7,9-26].
CHARACTERISTICS OF STUDIES INCLUDED IN THE FINAL ANALYSIS
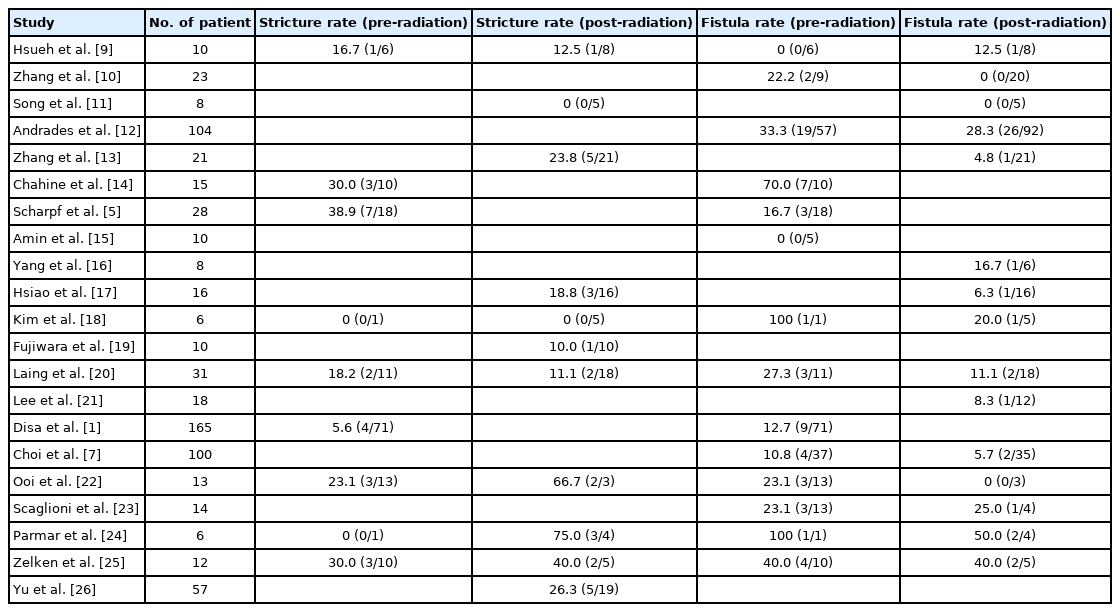
Among the 21 studies, we identified a total of 675 patients who underwent hypopharyngeal reconstruction and received perioperative radiation treatment. The clinical data and pooled analysis of the enrolled studies are shown in Table 1. In the enrolled studies, the stricture rate associated with preoperative and postoperative radiation treatment was 0% to 75%. The fistula rate associated with preoperative and postoperative radiation treatment was 0% to 100%. The mean stricture rate associated with preoperative and postoperative radiation treatment was 16.3% and 21.1%, respectively. The mean fistula rate associated with preoperative and postoperative radiation treatment was 22.5% and 16.1%, respectively.
THE EFFECTS OF PREOPERATIVE RADIATION TREATMENT
The stricture rate after preoperative radiation treatment was analyzed in six studies. A meta-analysis yielded a pooled odds ratio for stricture formation of 1.882 (95% confidence interval [CI], 0.756–4.684; p= 0.174) with low heterogeneity (Fig. 2). The fistula rate after preoperative radiation treatment was analyzed in 13 studies. A meta-analysis yielded a pooled odds ratio for fistula formation of 2.410 (95% CI, 1.240–4.686; p= 0.010) with low heterogeneity (Fig. 3). Fistula formation was shown to be significantly associated with preoperative radiation treatment.
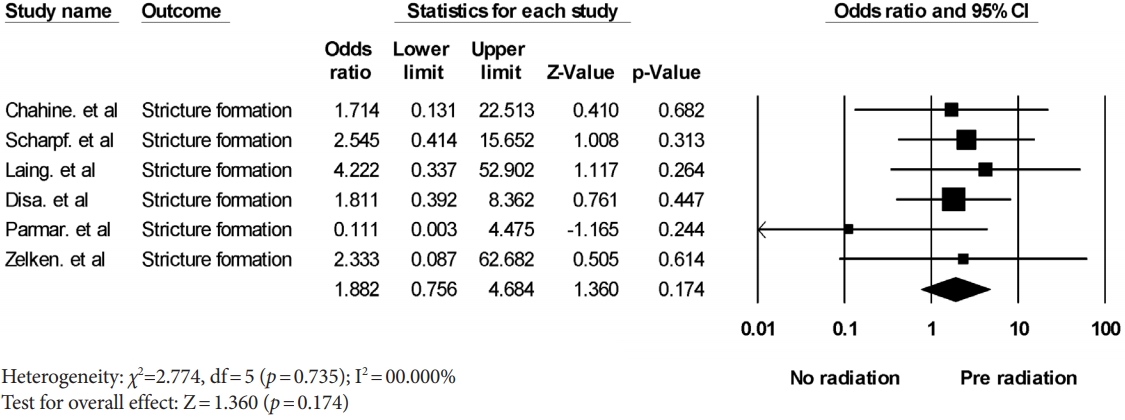
Forest plot of the stricture formation rate associated with preoperative radiation. The diamond indicates the summary estimate of the pooled studies. CI, confidence interval.
THE EFFECTS OF POSTOPERATIVE RADIATION TREATMENT
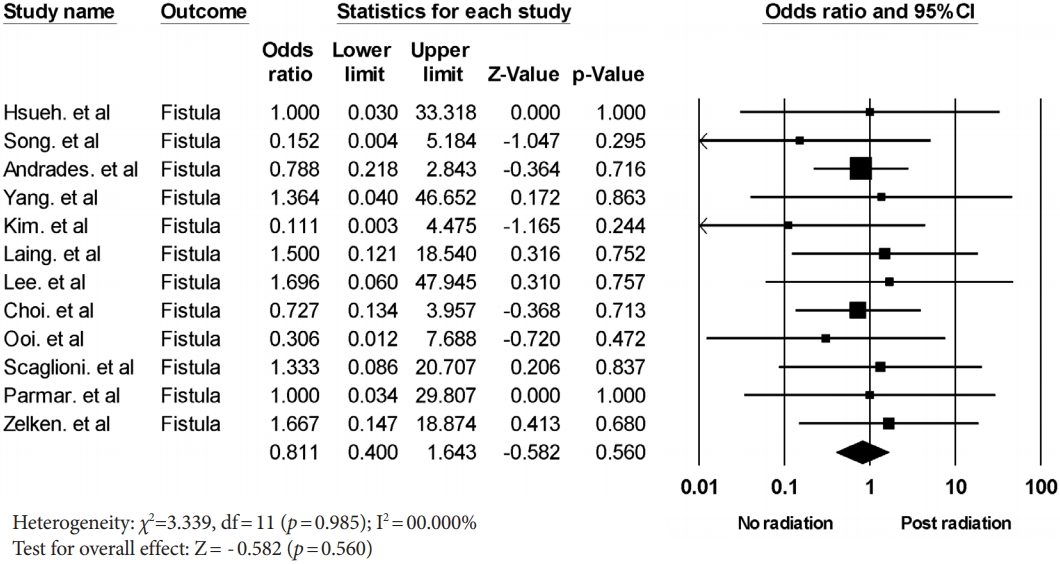
The stricture rate after postoperative radiation treatment was analyzed in six studies. A meta-analysis yielded a pooled odds ratio for stricture formation of 2.711 (95% CI, 1.029–7.140; p= 0.044) with low heterogeneity (Fig. 4). The fistula rate after postoperative radiation treatment was analyzed in 12 studies. A meta-analysis yielded a pooled odds ratio for fistula formation of 0.811 (95% CI, 0.400–1.643; p= 0.560) with low heterogeneity (Fig. 5). The stricture formation was shown to be significantly associated with postoperative radiation treatment.

Forest plot of the stricture formation rate associated with postoperative radiation. The diamond shape indicates the summary estimate of the pooled studies. CI, confidence interval.
INFLUENCE OF RADIATION TREATMENT
Additional radiation treatment after extensive dissection of the remnant esophagus makes the vascularity worse, and it can lead to fibrosis and stricture. The effect of radiation on stricture formation is a controversial issue. Some authors suggest that radiation and stricture formation are not related [27,28]. However, other studies identified that there is a significant association of radiation with stricture formation [29,30]. Perhaps, stricture induction could be dependent on the dose of radiation. A radiation dose greater than 50–60 Gy may possibly cause stricture formation [31-33]. Typically, the total dose of postoperative radiation was more than 60 Gy in our enrolled studies [9,11]; therefore, the results of our meta-analysis support a meaningful relationship between postoperative radiation and stricture formation.
The effect of radiation on wound healing is somewhat well known. Microvascular thrombosis by radiation can lead to impaired wound healing and increased tissue friability [34]. This vessel injury is associated with diminished smooth muscle density and fibrosis of the vessel wall [35,36]. Although the impaired wound healing state induced by radiation may be considered to cause fistula formation, some studies stated that there was not a significant association between radiation and fistula formation [37,38]. Our study verified that preoperative radiation treatment is significantly associated with fistula formation. The reason why postoperative radiation may not be associated with fistula formation is that fistula formation is a rather early complication, and the radiation effect is typically seen 3 months after completion of radiation treatment [32].
LIMITATIONS AND CONCLUSION
This study is a review study investigating the effect of perioperative radiation treatment on stricture and fistula formation in patients with hypopharyngeal reconstruction. The thorough search for the enrolled studies and robust meta-analysis strengthen this study. On the other hand, our study has several limitations. First, we could not analyze the detailed effect of radiation according to the method used because almost no studies stated the exact parameters of radiation such as the total dose, duration, etc. Therefore, this analysis can provide important indicators that support the need for further study. Second, some stated that the water-tight exquisite suture technique could prevent fistula formation [12]. However, due to the nature of the meta-analysis, we had to use extraction data from the results of many different surgeons. In addition, more randomized or prospective studies should be conducted to verify the radiation effect on postoperative complications with long-term follow-up.
In conclusion, preoperative and postoperative radiation treatment had a significant effect on the fistula and stricture formation rate at the hypopharyngeal reconstruction site. Depending on the use of perioperative radiation treatment, this study can support the surgeon’s ability to predict and prevent postoperative complications.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contribution
Conceptualization: SYL. Data curation: SYL. Formal analysis: SYL, SGR. Methodology: JYS. Project administration: JYS. Visualization: JYS, SGR, NHL. Investigation: JYS. Resources: JYS. Software: SGR, NHL. Writing - original draft: JYS. Writing - review & editing: JYS.