 |
 |
- Search
| Arch Craniofac Surg > Volume 22(6); 2021 > Article |
|
Abstract
Background
Methods
Results
Conclusion
Notes
Ethical approval
The study was approved by the Institutional Review Board of Hanyang University Seoul Hospital (IRB No. 2020-11-032) and performed in accordance with the principles of the Declaration of Helsinki. The informed consent was waived because this study design is a retrospective review.
Patient consent
The patients provided written informed consent for the publication and the use of their images.
Author contribution
Conceptualization: Seong Oh Park, Hee Chang Ahn. Data curation: Won Young Koo, Seong Oh Park. Formal analysis: Won Young Koo, Seong Oh Park. Methodology: Seong Oh Park. Writing - original draft: Won Young Koo. Writing - review & editing: Won Young Koo, Seong Oh Park, Hee Chang Ahn. Software: Soo Rack Ryu. Supervision: Hee Chang Ahn. All authors read and approved the final manuscript.
Fig. 1.
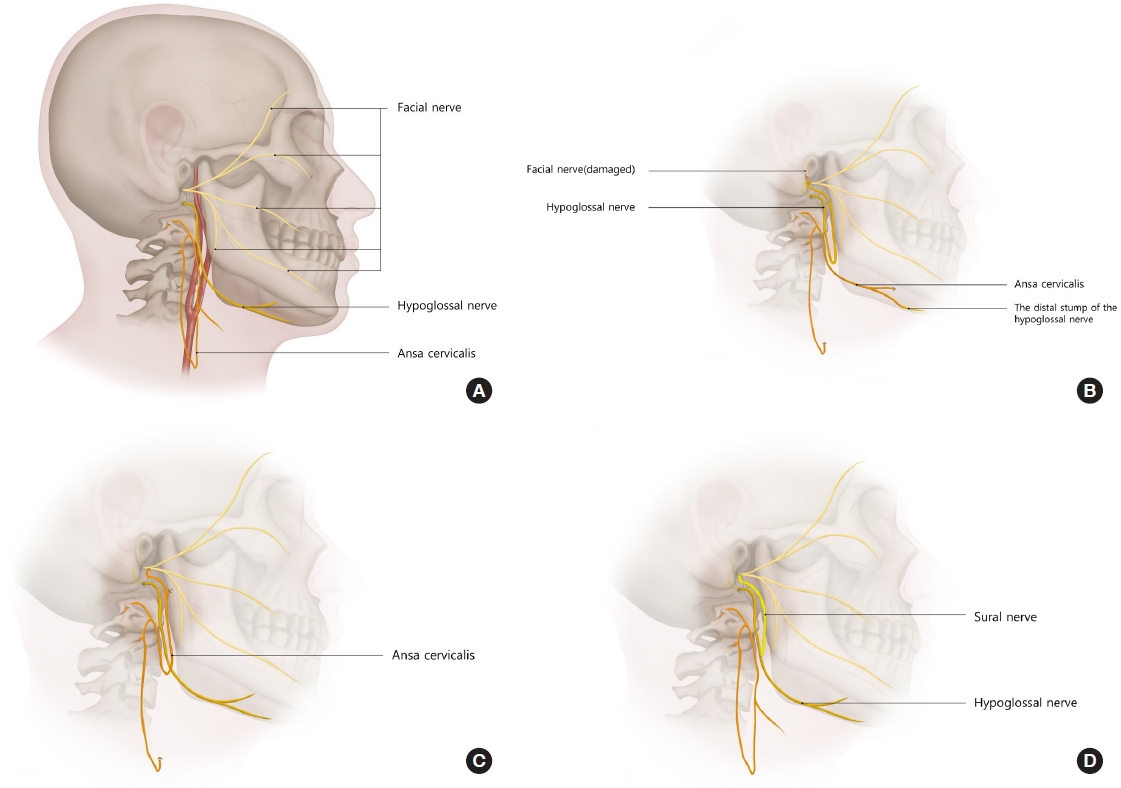
Fig. 2.
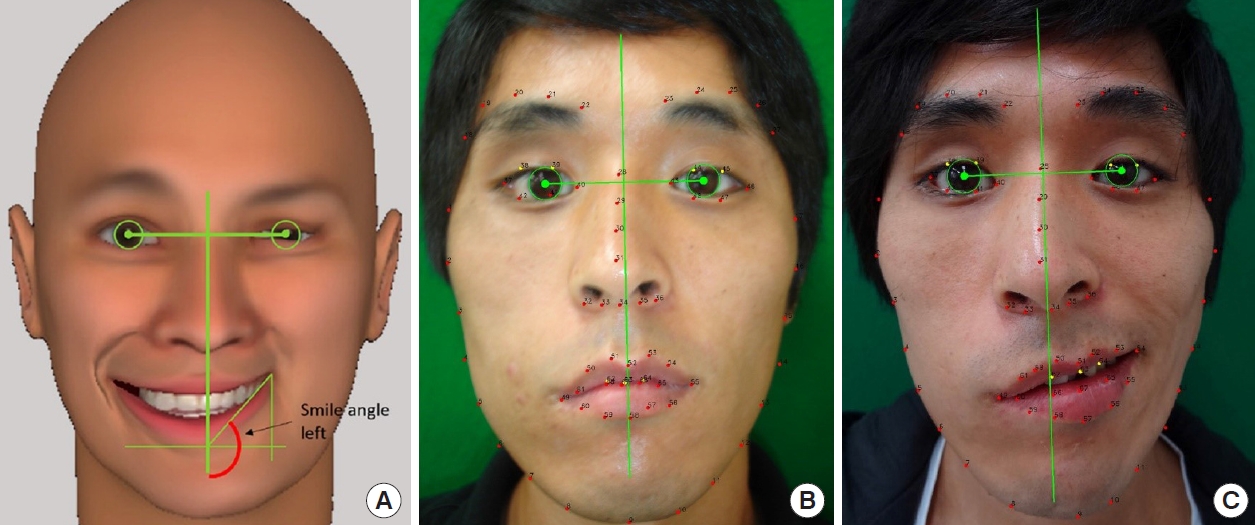
Fig. 3.

Fig. 4.
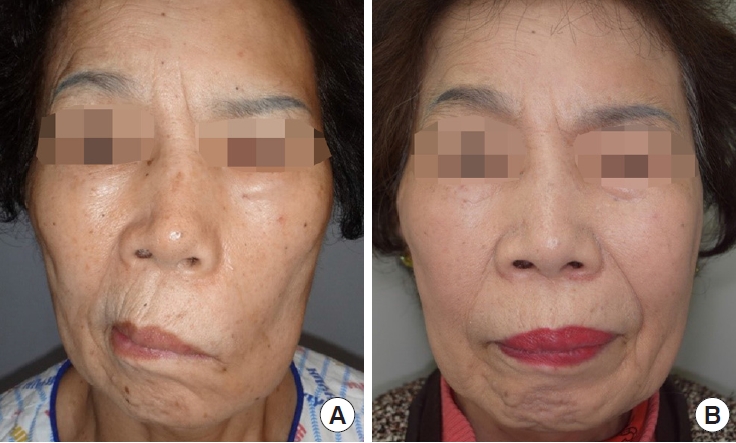
Fig. 5.

Fig. 6.
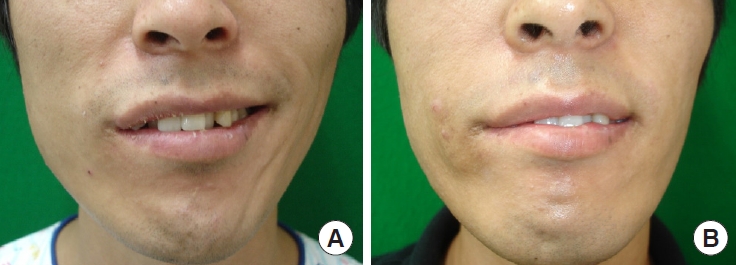
Table 1.
| Grade | Description |
|---|---|
| 1 | Normal |
| 2 | Discrete hemiatrophy, no deviation |
| 3 | Mild hemiatrophy, tongue deviation < 30˚ |
| 4 | Severe hemiatrophy, tongue deviation > 30˚ |
Reprinted from Martins et al. Neurosurgery 2008;63:310-6, with permission from Oxford University Press [11].
Table 2.
Table 3.
| Variable | Group 1 | Group 2 | Group 3 | p-valuea) |
|---|---|---|---|---|
| At rest (˚), mean±SD | ||||
| Preoperative | 9.96 ± 1.36 | 7.68 ± 1.27 | 8.25 ± 1.05 | |
| Postoperative | 3.47 ± 0.68 | 6.73 ± 1.48 | 6.89 ± 0.67 | |
| Change after surgery | 6.48 ± 0.77b) | 0.95 ± 0.53c) | 1.35 ± 1.02b),c) | 0.02 |
| While smiling (˚), mean±SD | ||||
| Preoperative | 18.02 ± 1.79 | 10.18 ± 1.03 | 9.15 ± 0.94 | |
| Postoperative | 4.14 ± 0.76 | 8.12 ± 0.72 | 7.92 ± 0.70 | |
| Change after surgery | 13.88 ± 2.00b) | 2.06 ± 0.67b),c) | 1.23 ± 0.56c) | 0.01 |
REFERENCES
- TOOLS
-
METRICS

-
- 6 Crossref
- Scopus
- 3,946 View
- 79 Download
- Related articles in ACFS






