Extraordinarily aggressive cutaneous sarcomatoid squamous cell carcinoma of the face: a case report
Article information
Abstract
Sarcomatoid squamous cell carcinoma (SSCC), a biphasic malignant tumor consisting of atypical squamous epithelial and mesenchymal elements mixed with epithelioid and spindle cells, is a variant of squamous cell carcinoma. Cutaneous SSCC is very rare and aggressive and has a poor prognosis. Here, we report a case of cutaneous SSCC with satellites and in-transit metastases. A 79-year-old woman presented with a protruding mass on the left temporal area sized 1.2×1.0 cm. The punch biopsy report indicated keratoacanthoma or well-differentiated squamous cell carcinoma. The size of the tumor increased to 2.7×2.0 cm after 8 days. An excisional biopsy was performed with a 2 mm safety margin. The tumor was identified as SSCC with a clear resection margin. Reoperation was performed thrice with an increased safety margin of 10 mm; however, the cancer recurred along with satellites and in-transit metastases. Chemoradiotherapy was administered; however, the size of the tumor increased along with satellites and in-transit metastases. The patient expired 162 days after the initial excision. Complete excision and immediate multidisciplinary approach should be combined during the early stages due to the aggressiveness and poor prognosis of cutaneous SSCC with satellites and in-transit metastasis.
INTRODUCTION
Sarcomatoid squamous cell carcinoma (SSCC), also known as spindle cell squamous cell carcinoma (SCC), is a rare variant of SCC. It shows a biphasic histological appearance consisting of a stromal element with invasive fusiform or sarcomatoid spindle cell proliferation and epithelial changes varying from dysplasia to invasive carcinoma [1]. A malignant tumor that exhibits biphasic morphology is termed tumor carcinosarcoma and is known by various names, such as pleomorphic carcinoma, pseudosarcoma, and sarcomatoid carcinoma [2].
Cutaneous SSCC is very rare, with only 52 cases reported in Korea between 1999 and 2018 (Table 1) [3]. Nomenclature, histological appearance, and scarcity can cause diagnostic and therapeutic problems [4]. Satellites and in-transit metastases are considered non-nodal locoregional metastases [5]. There are very few reports of these cases in cutaneous SCC [6-9]. Here, we report an exceptionally aggressive cutaneous case of SSCC with satellites and in-transit metastases.
CASE REPORT
A 79-year-old woman presented with a 1.2×1.0 cm rubbery and crater-like mass in the left temporal area (Fig. 1). Two years ago, the patient had undergone laser ablation treatment for a small polyp without biopsy at a private clinic; however, it had recurred 1 month before visiting our hospital. Punch biopsy suggested keratoacanthoma or well-differentiated SCC. Two weeks later, at the time of surgery, the lesion grew to 2.7×2.0 cm (Fig. 2). Due to the possibility of invasive SCC, the necessity of wide excision and the possibility of damage to the temporal branch of the facial nerve were explained. However, at the patient’s request, after an excisional biopsy with a safety margin of 2 mm, it was decided to perform additional surgery according to the biopsy results.

A 79-year-old woman with a 1.2 × 1.0 cm rubbery, firm, protruding, crater-like mass on the left temporal area.

Photograph on the first surgery (excisional biopsy) day. Two weeks after the first visit, the original mass grew to 2.7×2.0 cm. The safety margin was 2 mm, and the base was the subcutaneous layer. The margins were clear; however, the base margin was too close to the cancer (933 µm).
Microscopic examination revealed a well-differentiated SCC epithelial and poorly differentiated sarcoma-like mesenchymal components. Some areas exhibited a transition from squamous to spindle cells (Fig. 3). During immunohistochemical staining, the SCC component tested positive for cytokeratin and p63, and the sarcomatous region tested positive for p63 and vimentin, confirming the diagnosis of SSCC.

Microscopic examination of the first surgery shows the biphasic, malignant nature of the tumor. A focal area shows the cobblestone-like epithelial component gradually transits to or mixes with the elongated shape of the sarcomatoid element (H&E, ×100).
The resection margin was free from malignancy; however, the base resection margin was only 933 μm from the malignancy. Additional surgery for complete tumor resection with adequate safety margins and subsequent flap coverage was considered necessary. However, despite explaining the necessity of the procedure, the patient-declined complete resection; finally, wide excision and skin grafting were performed at the patient’s request. Computed tomography (CT) of the neck and chest was performed to detect potential metastases. No regional lymph node enlargement was identified. Preoperative chemotherapy, radiotherapy, and further evaluation, including magnetic resonance imaging or positron emission tomography-CT, were not administered due to the unwillingness of the elderly patient. Seven days before the second surgery, a 1.0×0.5 cm rubbery and protruding mass recurred. The tumor grew to 2.0×1.5 cm on the day of the surgery.
The second surgery was performed 42 days after the first surgery. A wide excision was performed above the deep temporal fascia with a safety margin of 5 mm. A permanent biopsy confirmed all margin involvement with malignancy. A third surgery for complete excision was planned. Tumor recurrence at the surgical site, accompanied by satellites and in-transit metastases in the neighboring areas, was observed 7 days before the complete excision (Fig. 4). The masses enlarged aggressively, the satellite masses merged into a large mass, and the overall affected area, including the in-transit metastases, was 7.0×9.0 cm (Fig. 5).

Photograph 63 days after the first surgery (7 days before the third surgery). Recurrence at the surgical site and several satellites and in-transit metastases in the neighboring region were observed after the removal of the tumor using wide excision. Each metastasis was approximately 1 cm in size, and it possessed rubbery, firm, protruding characteristics.
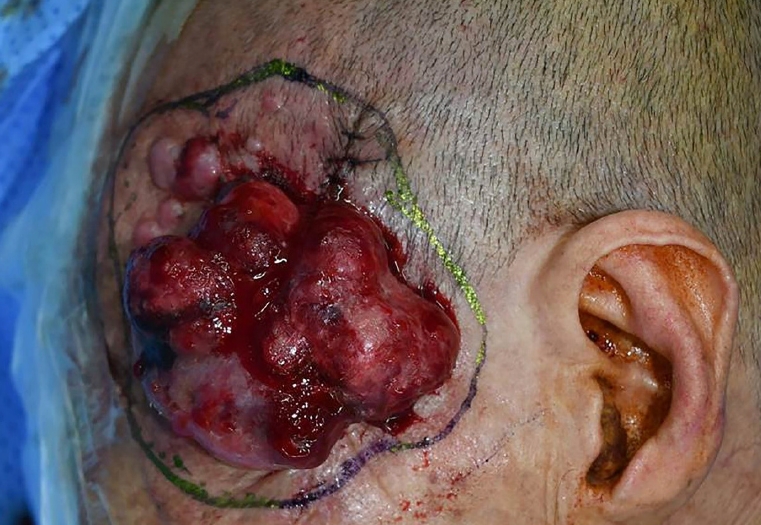
Preoperative photograph taken on the day of the third surgery (70 days after the first surgery). Within 7 days of the discovery of the satellites and in-transit metastases, the satellites merged to grow to 7.0×5.0 cm. The in-transit metastasis grew in size as well. The affected area is estimated to be 7.0×9.0 cm, and ulcerative bleeding was observed. The third surgery was performed to complete the excision, including the deep temporal fascia and periosteum. The temporal branch and the zygomatic branches of the facial nerves were resected.
The third surgery was performed 28 days after the second surgery. Complete resection was performed with a safety margin of 10 mm, including deep temporal fascia and periosteum. All margins were free from malignancy according to the biopsy, and chemo- and radiotherapy were planned 6 weeks after the surgery. We obtained CT images of the neck, chest, abdomen, and pelvis, which showed multifocal cluster nodular lesions in the left parotid gland and lungs. Furthermore, 3 weeks after the complete excision, small bean-like masses recurred at the primary lesion site. The masses doubled in size 4 days after their discovery (Fig. 6). A fourth surgery for additional wide excision was planned before the chemotherapy.
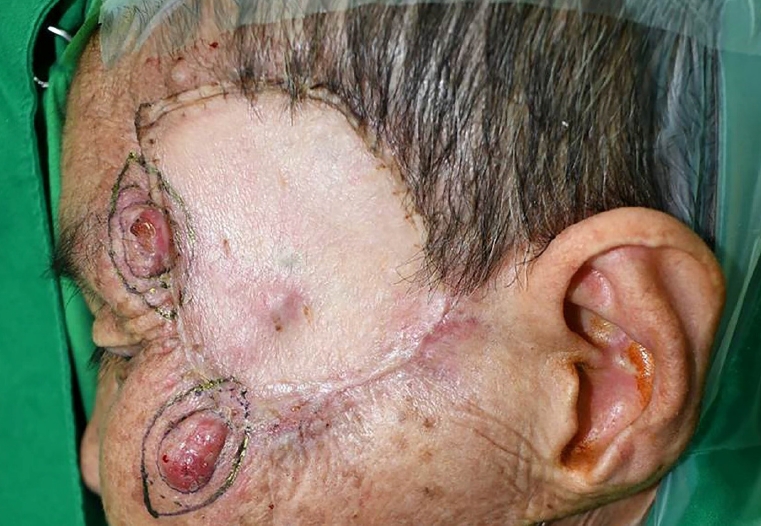
Preoperative photograph taken on the day of the fourth surgery (96 days after the first surgery). The recurrent masses, initially 0.5×0.5 cm, had grown to 2.0×1.0 cm. Additional wide excision and full-thickness skin graft were performed for the two recurrent masses. Except for the two sites where the design of excision was made, small masses were seen in the center of the previous skin graft site and at 12 o’clock. However, the patient rejected excision of the small masses.
The fourth surgery was performed 26 days after the third surgery. Wide excision was performed with a safety margin of 5 mm, including additional deep temporal fascia and periosteum, and a frozen biopsy was used to confirm that the margins were free from malignancy. Fourteen days after the surgery, cisplatin and doxorubicin were administered every 3 weeks as part of the chemotherapy regimen, and palliative radiotherapy (30 Gy in 10 daily fractions of 3 Gy each) was commenced 42 days after the surgery. However, a week after the surgery, several satellites were observed around the surgical site. Two weeks after the surgery, the total affected area reached 18×15 cm. The masses invaded the left upper eyelid; therefore, the patient was unable to open the eye (Fig. 7). Despite chemotherapy and radiotherapy, the mass kept growing and invaded the scalp and the nose 4 weeks after the surgery and further proliferated towards the scalp and the neck by creating numerous satellites (Fig. 8).

Photograph 110 days after the first surgery (2 weeks after the fourth surgery). Recurrent masses and satellites grew along with the margin of the reconstruction area. The size of the affected area reached 18×15 cm. Increasingly rapid growth of the tumor, along with the ulcerative lesion, was observed.

Photograph 124 days after the first surgery (4 weeks after the fourth surgery). The size of the mass reached 35×25 cm 4 weeks after the fourth surgery. Despite receiving chemotherapy and radiotherapy, the satellites and in-transit metastases rapidly grew.
The patient passed away 67 days after the final surgery. It had been 100 days after the satellites, and in-transit metastases were first observed.
DISCUSSION
Sarcomatoid (spindle cell) carcinomas are unusual variants of SCC, accounting for 3% of all squamous carcinomas in the head and neck region [10]. They predominantly arise in visceral organs (breast, lung, gastrointestinal tract, kidney, urinary bladder, thyroid, and others) and rarely occur as primary cutaneous tumors [11].
Sarcomatoid carcinoma shows biphasic morphology consisting of epithelioid and spindle-shaped neoplastic cells [1]. There are several variations of biphasic morphology, which include mixtures of SCC with sarcomatoid spindle cells, basal cell carcinoma, and true mesenchymal sarcoma [4]. There are several hypotheses for the expression of this biphasic morphology. The most widely supported theory states that the tumor is of an epithelial origin and undergoes de-differentiation as it shows monoclonal origin from stem cells [12]. In terms of microscopic and immunohistochemical findings, this transformation of epithelial cells into spindle cells is called epithelial-mesenchymal transition (EMT), which was observed in this case [10]. EMT is the process by which epithelial cells adopt a mesenchymal phenotype or fibroblast-like properties [13]. During EMT, differentiated epithelial cells lose their characteristics, including cell adhesion and polarity; reorganize their cytoskeleton; and acquire spindle cell morphology, stretch, break connections, and migrate [13]. Therefore, in the treatment of suspected sarcomatoid carcinoma, a microscopic examination should be conducted, and the morphological change from epithelial cell characteristics (cobblestone-like and nonmotile) to mesenchymal cell characteristics (elongated, motile, and invasive) should be confirmed [13]. However, in many cases of sarcomatoid carcinoma, spindle cells or pleomorphic cells are observed without clear epithelial differentiation during the microscopic inspection, which hinders the diagnostic differentiation of sarcomatoid carcinoma from primary spindle cell sarcoma, melanoma, and reactive spindle cell lesions [14]. In such cases, immunohistochemical staining can be useful as the biomarker of epithelial differentiation.
Cytokeratin, vimentin, s-100, desmin, carcinoembryonic antigen, smooth muscle actin, and epithelial membrane antigen are often used in staining [15]. SCC of epithelioid is specific to cytokeratin and p63 [16]. Sarcomatoid carcinoma tests are positive for vimentin and negative for other biomarkers [2,13].
Generally, survival rates of cutaneous carcinosarcomas are not as high as that of SCC. One study reports that the 5-year disease-free rates of adnexal-derived carcinosarcomas and epidermal-derived carcinosarcomas are 25% and 70%, respectively [17]. Poor prognostic factors for carcinosarcoma in decreasing order of significance include recent tumor growth, regional lymph node metastasis, history of long-standing skin tumor (>3 years), presence of adnexal differentiation, young age (<65 years), and tumor size (>2 cm) [17]. In this case, the presence of satellites and in-transit metastases were confirmed before regional lymph node metastasis. The notions of satellites and intransit metastasis are derived from melanoma, implying nonnodal metastasis [5]. Satellites are cutaneous or subcutaneous metastases located within 2 cm of the primary tumor margin. In-transit metastases are located more than 2 cm from the margin but are in the same region as the primary tumor or echelon of the regional lymph node region [5]. Melanoma associated with satellites or in-transit metastasis is classified as node C, which signifies stage IIIB or more advanced stages, and its 5-year overall survival rate is under 83% [5]. Satellites or intransit metastasis are not criteria for TNM staging for SCC. However, the prognosis for SCC accompanied by in-transit metastasis is very poor, with a 5-year overall survival rate of 13% [7]. These phenomena represent the aggressiveness of the primary disease. Satellites or in-transit metastatic lesions microscopically appear as sharply circumscribed nodules with a clear demarcation [4]. Therefore, the standard local treatment for cutaneous SSCC is complete excision of all the affected areas rather than wide excision of the primary lesion and each metastasis [4]. Surgical treatment with a 10 mm free circumferential margin, including deep excision to the fascia, is recommended [11].
There is no high-level evidence for the benefits of adjuvant chemo- or radiotherapeutic treatment on the sarcomatous part of the cutaneous SSCC [11]. However, there are arguments that chemotherapy [17] or radiotherapy [18] may be necessary [19].
Cutaneous SSCC is a very rare type of cancer with unknown causes, ambiguous clinical manifestation, and a poor prognosis, and its accompaniment with in-transit metastasis or satellites is even rarer [11]. In this case, it was not ideal that the intervals between the surgeries were long. In addition, considering the invasiveness of the primary tumor, it cannot be excluded that local seeding or metastasis of the tumor cells existed during the surgical procedure before complete excision. Furthermore, the rapid proliferation of the satellites and in-transit metastases and the delay in the chemo- or radiation treatment due to the consultation advice and repeated surgeries adversely affected the prognosis. Radiation therapy after the first operation was not performed due to the patient’s refusal. The second surgery was postponed during an evaluation to identify a benign thyroid mass found on the neck CT and to visit another general hospital. After the second surgery, radiation therapy was planned again, but in-transit metastasis occurred while the patient visited a tertiary hospital and returned. Radiation therapy after the third surgery was delayed according to the consultation advice that it can be performed 6 weeks after the skin graft. The prognosis could have been improved if the patient had undergone complete tumor resection and accepted chemotherapy and radiation therapy during the early stages. Locoregional recurrence is a risk factor for distant metastasis, and therapy for satellites and in-transit metastasis in SSCC is less standardized [20]. Therefore, it is necessary to more aggressively explain the invasiveness of SSCC at the early detection and to contemplate a process that involves local, regional, or systemic analysis and treatment options for an immediate multidisciplinary approach.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was approved by the Institutional Review Board of Daegu Fatima Hospital (IRB No. DFE21ORIO105).
Patient consent
The patient provided written informed consent for the publication and use of her photographs.
Author contribution
Conceptualization: Yunjae Lee, Hyeonjung Yeo, Hannara Park, Hyochun Park. Data curation: Yunjae Lee, Dongkyu Lee, Hyochun Park. Formal analysis: Yunjae Lee, Hyochun Park. Project administration: Hyochun Park. Writing - original draft: Hyochun Park. Writing - review & editing: Hyochun Park. Resources: Hyochun Park.
Abbreviations
CT
computed tomography
EMT
epithelial-mesenchymal transition
SCC
squamous cell carcinoma
SSCC
sarcomatoid squamous cell carcinoma

