Surgical outcomes of suprafascial and subfascial radial forearm free flaps in head and neck reconstruction
Article information
Abstract
Background
Conventional radial forearm free flaps (RFFFs) are known to be safe, but can result in donor site complications. Based on our experiences with suprafascial and subfascial RFFFs, we evaluated the safety of flap survival and surgical outcomes.
Methods
This was a retrospective study of head and neck reconstructions using RFFFs from 2006 to 2021. Thirty-two patients underwent procedures using either subfascial (group A) or suprafascial (group B) dissection for flap elevation. Data were collected on patient characteristics, flap size, and donor and recipient complications, and the two groups were compared.
Results
Thirteen of the 32 patients were in group A and 19 were in group B. Group A included 10 men and three women, with a mean age of 56.15 years, and group B included 16 men and three women, with a mean age of 59.11 years. The mean defect areas were 42.83 cm² and 33.32 cm², and the mean flap sizes were 50.96 cm² and 44.54 cm² in groups A and B, respectively. There were 13 donor site complications: eight (61.5%) in group A and five (26.3%) in group B. Flexor tendon exposure occurred in three patients in group A and in none in group B. All flaps survived completely. A recipient site complication occurred in two patients (15.4%) in group A and three patients (15.8%) in group B.
Conclusions
Complications and flap survival were similar between the two groups. However, tendon exposure at the donor site was less prevalent in the suprafascial group, and the treatment period was shorter. Based on our data, suprafascial RFFF is a reliable and safe procedure for reconstruction of the head and neck.
INTRODUCTION
The radial forearm free flap (RFFF) was developed by Dr. Yang in the 1970s and continues to be used to reconstruct various types of defects [1,2]. The RFFF is a thin, pliable flap that is easily dissected and exhibits few pedicle variations; thus, it is commonly used for head and neck reconstruction (e.g., treatment of partial tongue defects, or esophagus reconstruction) [3,4]. However, several donor site morbidities such as delayed healing, tendon exposure, infection, skin graft loss, and abnormal sensation have been reported [5-7]. Notably, it has been reported that an exposed flexor carpi radialis (FCR) is difficult to treat and requires prolonged treatment.
Since Webster and Robinson [8] introduced the suprafascial radial forearm flap in 1995, several methods have been tried to maintain flap stability and reduce donor site morbidity. In this study, we sought to determine whether the suprafascial elevation is a reliable method by comparing postoperative donor and recipient site complications of suprafacial and subfascial methods after head and neck reconstruction.
METHODS
This retrospective study analyzed patients who underwent RFFFs for head and neck reconstruction from April 2006 to June 2021. The surgical methods differed depending on operator preference. Two operators performed subfascial dissections. Another operator performed subfascial dissections from 2012 to 2015 and suprafascial dissections after 2015. In total, 32 patients were included in this study and were classified into two groups according to the RFFF flap elevation method used. Group A included patients who underwent subfascial RFFF, and group B included patients who underwent suprafascial RFFF. The data collected for analysis included patient demographics, age, medical history, smoking history, reconstruction site, cancer type, flap size, defect characteristics, and follow-up duration.
In group A, flap elevation was started under an air tourniquet with a 250-mmHg pressure from the ulnar side, and subfascial dissection was performed from the flexor carpi ulnaris (FCU) muscle fascia, approaching the FCR. The elevated flaps included the paratendon of the FCR. On the radial side, fascia of the brachioradialis (BR) was dissected. If necessary, the palmaris longus was included in the flap, but was otherwise left off. The radial artery was identified and dissected between the FCR and BR, and the flap was harvested according to the required flap size and pedicle length (Fig. 1A). In group B, under an air tourniquet with a 250-mmHg pressure, the muscle fascia of the FCU on the ulnar side was left, and the dissection was performed to the FCR between the subcutaneous fat and fascia while leaving the FCR paratendon. During suprafascial flap elevation, the palmaris longus was included in the flap when flap support or making a vocal cord was needed. On the radial side, suprafascial dissection was performed on the BR (Fig. 1B). The flap was then harvested according to the required pedicle length and flap size. In both groups, the donor site was covered with a split-thickness skin graft (STSG). Acellular dermal matrix (ADM) (AlloDerm, LifeCell Corp.; SureDerm, Hans Biomed Corp.; Matriderm, MedSkin Solutions Dr. Suwelack AG) was also used with consent in some patients. A tie-over dressing was used on donor sites and removed on postoperative day 7, with flap monitoring continued until postoperative day 14. Flap survival and donor and recipient site complications were evaluated at 4 weeks postoperatively. A complication was defined by the need for a dressing or procedure at the 4-week follow-up due to delayed wound healing. The end point of treatment at the donor site was when all epithelialization was completed and additional dressing was not required.

Subfascial and suprafascial dissection methods of radial forearm free flaps: (A) subfascial dissection (arrow: flexor carpi radialis tendon) and (B) suprafascial dissection (arrows: remaining fascia).
We compared the incidence of donor and recipient site complications in the two groups to the incidence of complications found in previous relevant reports. Data analysis was performed using SPSS version 20.0 (IBM Corp.) and SAS version 9.4 (SAS Institute). Intergroup comparisons were made using the two-sample t-test for the continuous variables, and the chi-square test or Fisher exact test for the categorical variables. Statistical significance was accepted for p-values < 0.05 (two-tailed).
RESULTS
Patient demographic data
The 32 patients who underwent head and neck reconstruction using RFFFs were allocated to either group A (n = 13; subfascial dissection) or group B (n = 19; suprafascial dissection). All patients in group A had squamous cell carcinoma. In group B, one patient had mucoepidermoid carcinoma, and all other patients had squamous cell carcinoma.
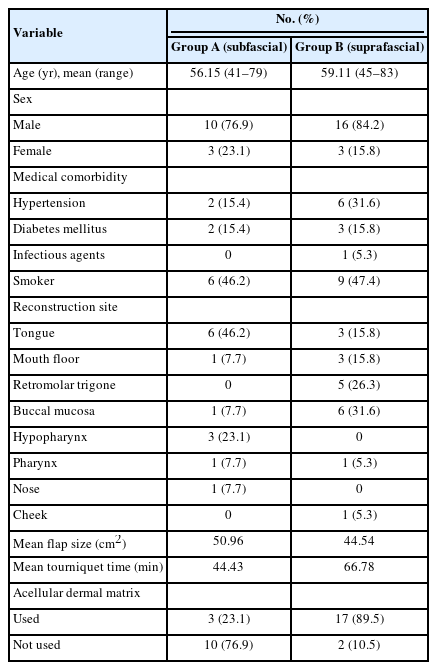
In group A, there were 10 men (76.9%) and three women (23.1%) with a mean age of 56.15 years (range, 41–79 years). Reconstruction sites included the tongue in six patients (46.2%), the hypopharynx in three patients (23.1%), and the mouth floor, buccal mucosa, pharynx, and nose in one patient (7.7%). The mean defect size was 42.83 cm² (range, 15.75–65 cm²) and the mean flap size was 50.96 cm² (range, 36–65 cm²). Medical comorbidities included hypertension in two patients (15.4%), and diabetes mellitus in two patients (15.4%). Six patients (46.2%) were smokers. In group A, Alloderm and Surederm were used at the donor sites of three patients (23.1%) (Table 1). The mean follow-up was 8 months postoperatively.
Patient demographics in a comparative study of subfascial and suprafascial radial forearm free flaps
In group B, there were 16 men (84.2%) and three women (15.8%), with a mean age of 59.11 years (range, 45–83 years). The reconstruction sites were the buccal mucosa in six patients (31.6%), the retromolar trigone in five patients (26.3%), the mouth floor and tongue in three patients (15.8%), and the pharynx and cheek in one patient (5.3%). The mean defect size was 33.33 cm² (range, 16.5–66.5 cm²) and the mean flap size was 44.54 cm² (range, 20–78 cm²). Medical comorbidities included hypertension in six patients (31.6%), and diabetes mellitus in three patients (15.8%). Nine patients (47.4%) were smokers. Matriderm was used at the donor sites of 17 patients (89.5%) (Table 1). The mean follow-up was 8 months postoperatively.
There were no statistically significant differences between groups A and B regarding defect sizes (p = 0.078) or flap sizes (p = 0.21). There were nine patients in group A and 13 in group B for whom the tourniquet time was recorded. The mean tourniquet time was 44 minutes and 26 seconds in group A and 66 minutes and 47 seconds in group B, a statistically significant difference (p = 0.021).
Donor site complications
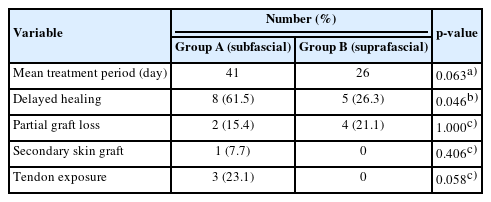
The mean donor site treatment period was 41 days in group A and 26 days in group B, a nonsignificant difference (p = 0.063). Delayed donor site wound healing at 4 weeks postoperatively occurred in 13 patients—eight patients (61.5%) in group A and five patients (26.3%) in group B (Table 2)—and showed a statistically significant difference (p = 0.046). In group A, partial graft loss and FCR tendon exposure occurred in two and three patients, respectively. Two patients with partial graft loss required an additional skin graft (Fig. 2A), and for the three patients with FCR tendon exposure, the exposed area was covered with a local advancement flap. Four patients in group B had partial graft loss, but there was no case of tendon exposure, and the remaining 15 patients in group B were well healed (Fig. 2B).

(A) Partial graft loss after subfascial dissection. (B) Wellhealed donor site after suprafascial dissection.
Of the above-mentioned donor site complications, the incidence of tendon exposure was marginally significant in both groups (p = 0.058). However, partial graft loss and secondary skin graft loss did not occur at significantly different rates (p = 1.000 and p = 0.406, respectively) (Table 2).
Recipient site complications
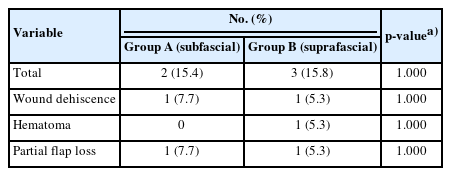
In group A, wound dehiscence occurred in one patient (7.7%). In group B, there were three (15.8%) complications, including wound dehiscence in one patient (5.3%), hematoma in one patient, and partial flap loss in one patient (5.3%) (Table 3). The incidence of recipient site complications in the two groups was not significantly different: total (p = 1.000), wound dehiscence (p = 1.000), hematoma (p = 1.000), and partial flap loss (p = 1.000) (Table 3).
DISCUSSION
RFFF is widely used for head and neck reconstruction because it has several advantages, such as a thin flap size, a consistent anatomy, and a lack of variation in the radial artery and concomitant vein. However, although subfascial RFFF is a conventional method, it has been reported to have high donor site complications, including delayed healing, tendon exposure, hematoma, and infection [6,7]. Flaps are usually harvested to include the fascia, because the deep fascial plane in the extremities has abundant blood flow, which is important for flap perfusion. Therefore, some studies have recommended including deep fascia [9,10]. Furthermore, a flap containing fascia maintain stable circulation and enable a bloodless and straightforward dissection. However, some studies have reported no problems with survival for flaps harvested with suprafascial dissection in the forearm and stated that the fascia of the muscle is not essential for the blood supply of the skin flap. They also suggest that the suprafascial RFFF is helpful in preventing donor site complications [11-13].
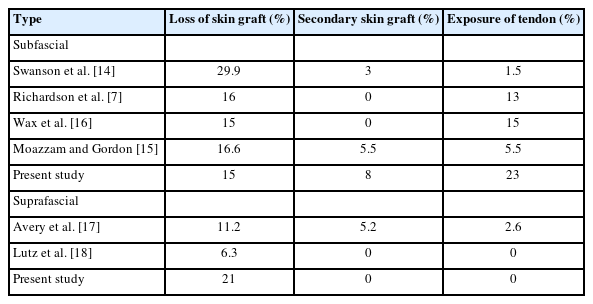
The donor site complications of this study were compared with the results of previous reports. The complications observed for subfascial RFFF were compared with those reported by Richardson et al. [7], Swanson et al. [14], Moazzam and Gordon [15], and Wax et al. [16], and the complications of suprafascial RFFF were compared with those reported by Avery [17] and Lutz et al. [18] (Table 4). In group A, the incidence of graft skin loss and secondary skin graft was similar to those previously reported, but the rate of tendon exposure was higher. In group B, the incidence of skin graft loss was higher and the incidence of tendon exposure and secondary skin graft were lower than those reported in previous studies.
Of all donor site complications, tendon exposure has been associated with poor outcomes and additional surgery and has been reported to occur more frequently after subfascial RFFF than suprafascial RFFF [7,9-10,14,17-18]. The incidence of tendon exposure for subfascial RFFF was reported to be 13% by Richardson et al. [7], 20% by Ghanem and Wax [19], and 15% by Wax et al. [16]. The incidence of FCR exposure after subfascial RFFF was reported by Avery [17] and Lutz et al. [18] to be 2.6% and 0%, respectively. In our study, the rate of tendon exposure was also lower for suprafascial RFFF, probably because there was less damage to the paratendon and intramuscular structures during flap elevation, and because the additional fascial layer suppressed tendon exposure after partial graft loss. In addition, the abundant vascular network in the remaining fascia formed a vascularized bed that likely increased graft survival by creating an appropriate gliding interface [10,20,21]. When the wrist and fingers move in flexion and extension, the fascia over the muscle is less mobile than the flexor muscle. Therefore, it appears that grafts on the fascia layer are more stable, which increases graft survival. Moreover, FCR tendon exposure occurred in many cases when donor site skin graft loss occurred after subfascial RFFF dissection. However, when donor site skin graft loss occurred after suprafascial RFFF dissection, tendon exposure did not occur due to the survival of the remaining paratendon. Therefore, the treatment period was short and a secondary STSG was possible. We thought that the ADM may have contributed to this result. ADM provides a template for the formation of neodermis tissue, which is inversely proportional to the extent of postoperative wound contracture [22]. Wester et al. [23] showed that, although the use of ADM was not associated with the incidence of complete graft loss at the donor site after suprafascial RFFF, the use of ADM was associated with a statistically significant increase in the frequency of partial graft loss. However, the use of ADM resulted in good secondary healing without a second STSG, whereas STSG alone resulted in more cases requiring a second STSG.
Traditionally, surgeons are concerned about possible flap loss due to poor flap circulation and stability when harvesting an RFFF without fascia. However, in the present study, all suprafascial RFFFs survived, and no serious donor or recipient site complications were encountered. The present study had several limitations that warrant consideration. Most obviously, the number of cases included was small, and the study was limited by its retrospective design. In addition, it was difficult to investigate the flap stabilities of suprafascial and subfascial RFFFs when flap size was maximized. Further research on suprafascial RFFFs is needed to improve our understanding of their usefulness.
This study showed that suprafascial RFFFs provided stable flap viability, reduced the need for additional surgery, decreased treatment duration, and decreased the incidence of tendon exposure, as compared with subfascial RFFFs. Based on these results, we consider the suprafascial RFFF to be an acceptable procedure in head and neck reconstruction.
Abbreviations
ADM
acellular dermal matrix
BR
brachioradialis
FCR
flexor carpi radialis
FCU
flexor carpi ulnaris
RFFF
radial forearm free flap
STSG
split-thickness skin graft
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Ethical approval
The study was approved by the Institutional Review Board of Inha University Hospital (IRB No. 2021-09-011).
Patient consent
The patients provided written informed consent for the publication and use of their images.
Author contributions
Conceptualization: Sae Hwi Ki. Writing - original draft: Tae Jun Park, Jin Myung Yoon. Writing - review & editing: Sae Hwi Ki. Resources: Sae Hwi Ki. Supervision: Sae Hwi Ki. All authors read and approved the final manuscript.
Acknowledgements
The statistical data used in this paper were supported by the Biostatistics Center of Inha University Hospital, Incheon, South Korea.