Basal cell adenoma of parotid gland: two case reports and literature review
Article information
Abstract
Most of salivary tumors are benign in nature and are typically diagnosed and classified based on their histopathological presentation. Basal cell adenoma of the salivary glands is a rare, benign disease accounting for 1% to 3% of salivary gland tumors. Despite its low incidence, basal cell adenoma is the third most common benign tumor of the salivary gland after pleomorphic adenoma and Warthin’s tumor. It usually appears as a firm and slow-growing mass. Due to the prognosis, differential diagnosis with basal cell adenocarcinoma, adenoid cystic carcinoma and basaloid squamous cell carcinoma is required. In this report, we present two cases; a 62-year-old woman who presented with an asymptomatic, and slow-growing mass and a 64-year-old woman with a static-sized mass in the parotid gland. In both cases, the mass was completely excised, postoperative pathology reports confirmed the diagnosis of basal cell adenoma. We also review the literature and discuss this rare entity.
INTRODUCTION
Basal cell adenoma (BCA) of the salivary glands is a rare benign disease that accounts for 1% to 3% of salivary gland tumors [1]. BCA is histologically similar to other tumors, such as pleomorphic adenoma, Warthin’s tumor, and basal cell adenocarcinoma (BCAC). Therefore, it is challenging to differentiate these diseases through fine needle aspiration (FNA), making superficial or total parotidectomy necessary for diagnosis and treatment [2,3]. Clinically, BCA is usually a slow-growing, asymptomatic, and movable mass. In this paper, we report rare but typical cases of BCA of the parotid gland. Monomorphic basaloid epithelial cell proliferation constitutes the histopathological hallmark of BCA. Typically, BCA is described as a slow-growing mass that is seldom symptomatic and is freely movable [4]. Adults between their fifth and seventh decades of life are most commonly affected. BCA carries a favorable prognosis in comparison with other salivary gland neoplasms, especially since recurrence is not common. Here, we report cases of the parotid mass presenting as an asymptomatic swelling histopathologically identified as BCA.
CASE REPORT
Case 1
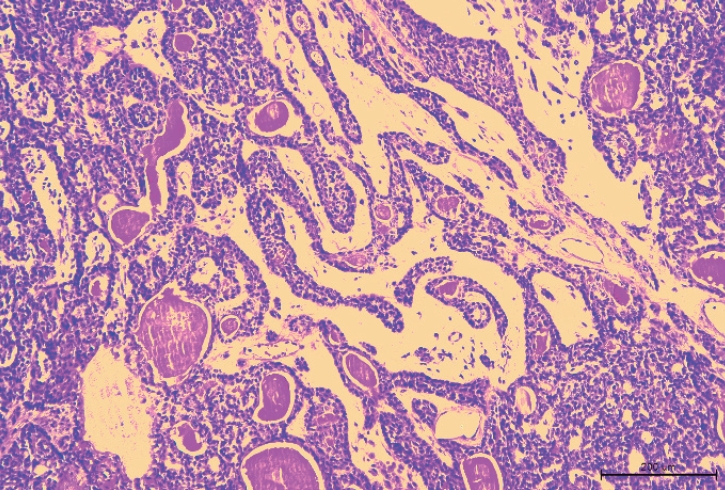
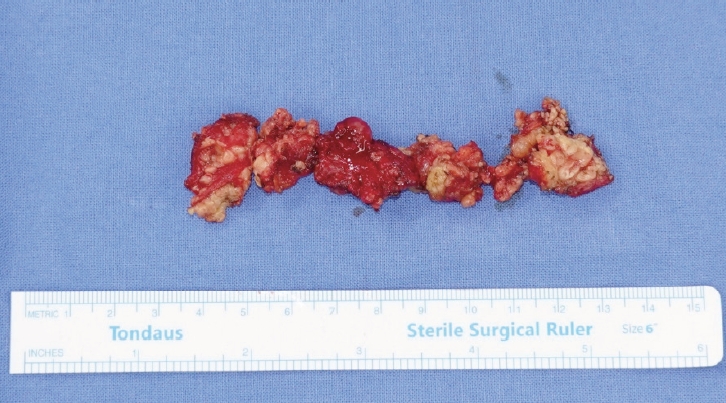
A 62-year-old woman visited the outpatient clinic for an asymptomatic mass on the left cheek that had gradually increased in size for 4 years. Computed tomography (CT) revealed a homogeneous and well-enhanced 1.5-cm sized nodule in the tail of the left parotid gland (Fig. 1, left). She was diagnosed with pleomorphic adenoma based on fine needle biopsy, and superficial parotidectomy was recommended, but the patient was lost to follow-up. Four years later, a follow-up CT was performed, and a 1.8-cm sized well-enhanced nodular mass was confirmed without lymph nodes metastasis (Fig. 1, right). The patient underwent total parotidectomy because the mass involved the deep lobe of the parotid gland (Fig. 2). Gross examination revealed an encapsulated mass without any cysts, necrosis, or invasion of surrounding tissues. All the facial nerve branches were preserved (Fig. 3). The final biopsy using hematoxylin and eosin staining revealed a trabecular pattern of basaloid cells separated by an eosinophilic basal membrane and small multiple cysts (Fig. 4). The patient did not experience any complications or recurrence until 2 years after the surgery.
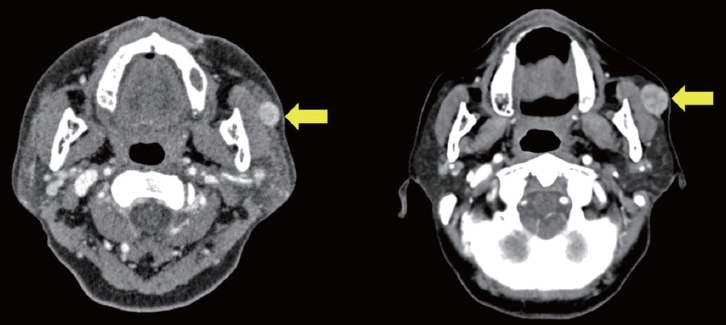
Preoperative enhanced computed tomography of case 1 patient with asymptomatic mass on the left cheek gradually increasing in size. The left one (4 years ago) shows a well-defined, homogeneous mass (yellow arrow) in the left parotid gland and a slightly larger mass with focal enhancement was observed in the right one (recently).
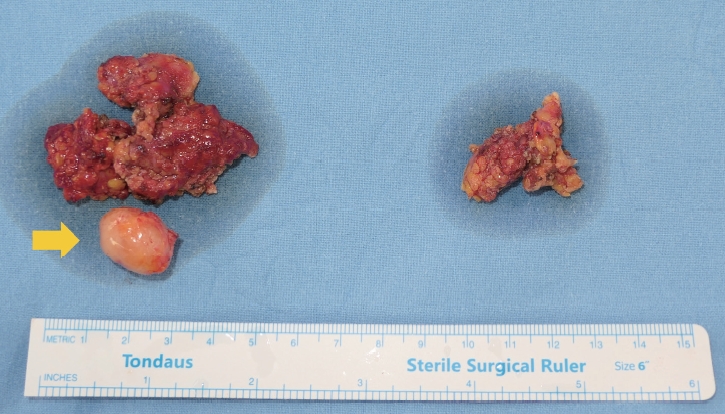
Intraoperative clinical photograph of the mass (yellow arrow) and parotid gland. On gross examination, a round, well-circumscribed, solid mass without necrosis was observed.
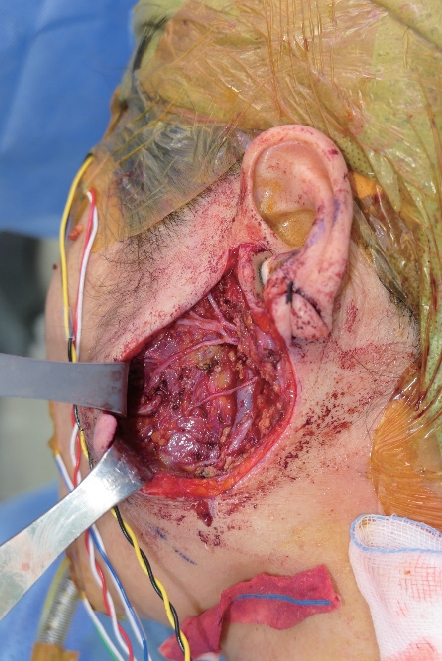
Intraoperative clinical photograph after total parotidectomy. All facial nerve branches were preserved.
Case 2
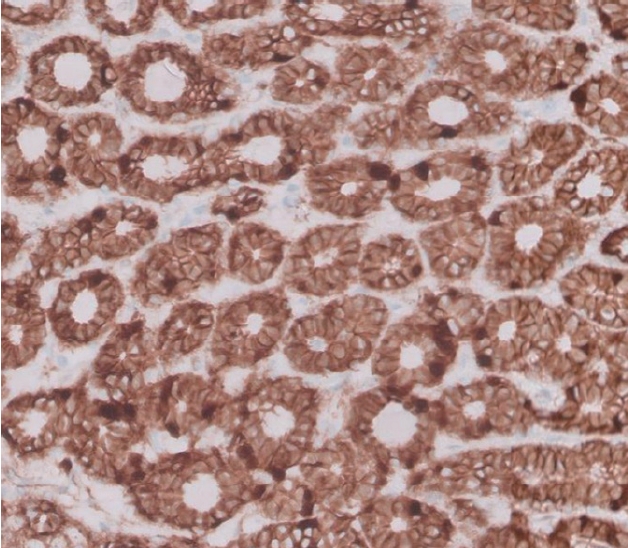
A 64-year-old woman was referred to the clinic by endocrinology due to a mass on her left cheek, which was accidentally found during the sonography of her annual follow-up for thyroid nodules. She discovered the mass on her left cheek mass 7 years ago. Ultrasonography revealed a 6 × 13 × 8 mm mass, and a FNA was performed, which diagnosed her with either BCA or cellular pleomorphic adenoma. Before the excision surgery, magnetic resonance imaging (MRI) was performed based on this diagnosis, which revealed a benign-looking, low-grade soft tissue mass in the anterior portion of the left parotid gland, such as a Warthin tumor or BCA/myoepithelioma (Fig. 5). She underwent superficial parotidectomy (Fig. 6). Gross examination revealed an encapsulated mass with the superficial lobe of the parotid gland. All the facial nerve branches were preserved (Fig. 7). Immunohistochemistry showed positive results for β-catenin nuclear staining, confirming the diagnosis of BCA (Fig. 8). The patient developed seroma on her left cheek and wound dehiscence after surgery, but it resolved with serial aspiration and simple closure under local anesthesia.
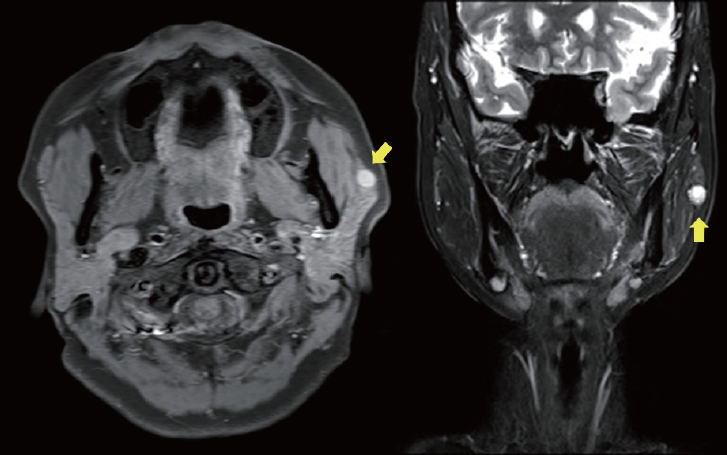
Preoperative enhanced magnetic resonance imaging (MRI) of case 2 patient (female/64, mass without size change for 7 years on left cheek, accidentally found on sonography of annual follow-up for thyroid nodules). MRI shows a benign-looking, low-grade soft tissue mass in the anterior portion, located in the superficial lobe of left parotid gland (yellow arrow).
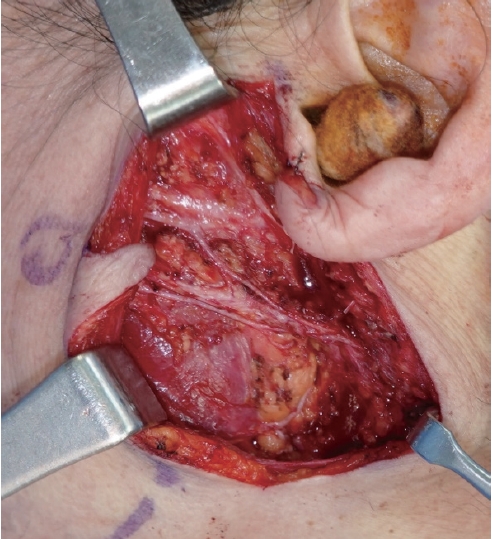
Intraoperative clinical photograph after superficial parotidectomy. All facial nerve branches were preserved.
LITERATURE REVIEW
BCA is a rare benign disease that arises from the basal cells of the salivary glands, accounting for 1% to 3% of salivary gland adenomas [4-6]. The condition is commonly observed in women aged over 50 years, and there is no specific known causative factor [7]. BCA usually develops in the parotid gland or minor salivary glands, but it can also occur in atypical locations such as the lips, buccal mucosa, palate, and nasal septum [8]. The tumors are slow-growing and typically asymptomatic, but tenderness, repeated nasal bleeding, or facial nerve compression due to the mass have been reported [9]. While they are typically benign, there is a small risk of recurrence if the entire tumor is not removed during surgery.
The diagnosis of BCA in the parotid gland involves a physical examination and imaging studies such as a CT scan or MRI. For tumors originating in the parotid gland, the extent of resection before surgery needs to be contemplated [10]. Membranous BCA, adenoid cystic carcinoma, pleomorphic adenoma, and BCA are all composed of basaloid cells. Therefore, cell cytology alone is not sufficient for diagnosis, even when considering differences in the ratio of cytoplasm. Some argue that core needle biopsy is helpful, but it may not be appropriate in cases involving hematoma and nerve damage. FNA is commonly used in conjunction with MRI. However, it is crucial to have a clinical suspicion, and even if the accuracy of FNA is low, the routine application of core biopsy and frozen section is not appropriate [11].
Preoperative imaging studies are also useful, as BCA shows contrast enhancement in a well-defined lesion on the early phase of enhanced CT [1]. It differs from pleomorphic adenoma, which shows contrast in the late phase with irregular margins, and from Warthin’s tumor, which occurs mainly in the caudal portion of the parotid gland in male smokers aged over 50 years [1]. As benign tumors of the parotid gland and their malignant counterparts have several overlapping features, a comprehensive interpretation based on clinical findings is necessary [12].
Histologically, BCA is composed of basaloid cells with a scanty pale cytoplasm and is characterized by the absence of a chondromyxoid stromal matrix. There are four histological patterns of BCA, namely solid, trabecular, tubular, and membranous types [13,14], with all types usually presenting a similar clinical prognosis. The recurrence rate for solid and trabecular-tubular variants is almost nonexistent. However, the membranous type is known to have a more progressive growth and higher recurrence rate, almost 25% [7,15]. The solid type is similar to the skin’s basal cell carcinoma with solid nests of basaloid cells surrounded by an outer layer of columnar/cuboidal cells. The trabecular type has basaloid cells arranged in narrow strands and cords separated by a fibrous and vascular stroma. The tubular type has basaloid cells and multiple small duct lumens lined by eosinophilic, cuboidal cells [16,17]. Immunohistochemistry has been studied for a more accurate diagnosis, and nuclear β-catenin expression is known to be common in BCA and BCAC, unlike other salivary gland tumors [18].
The differential diagnosis must include the pleomorphic adenoma, the most common benign tumor of the salivary glands, and other salivary gland tumors. BCAC, another malignant tumor sharing histologic features with the BCA, is distinguished from BCA by the histologic characteristics of invasion, mitotic activity, and neural or vascular involvement [19-21]. The transformation of BCA into BCAC is still controversial, but there is a report that BCA can transform into malignancy in up to 4.3% of cases [22]. However, most BCAs remain benign throughout their course. If malignant transformation of a BCA is suspected, additional testing and imaging may be necessary to confirm the diagnosis. BCAC, a malignant counterpart of BCA, is known to have a generally good prognosis due to low-grade malignancy. Radiotherapy may be required if the stage is ≥ T3 in patients aged over 65 years [23,24]. It has been found that BCA and BCAC have the same genetic modification, but whether BCA undergoes malignant transformation to BCAC has not been determined [25].
Recently, there has been an argument that enucleation can help reduce morbidity and improve facial aesthetics compared to parotidectomy in cases of clinically benign lumps, while still maintaining oncological safety [26]. However, for BCA of the parotid gland, parotidectomy remains the treatment of choice, and enucleation is not recommended due to higher rates of local recurrence. In cases where the tumor is located in the deep lobe, total parotidectomy is recommended as there is a high probability of malignancy [27,28].
DISCUSSION
Despite its low incidence, BCA is the third most common benign tumor of the salivary gland after pleomorphic adenoma and Warthin’s tumor. There has been recent debate regarding the oncological safety of enucleation for clinically benign lumps. However, considering the high recurrence rate of the membranous type of BCA, which can reach up to 25% even after enucleation, it is recommended to closely monitor patients and, if feasible, consider surgical removal beyond superficial parotidectomy. Additional reoperation may ultimately be necessary depending on the pathology type. Whenever possible, excision surgeries should aim for nerve sparing and include as much normal tissue as possible without damaging the capsule within the range of nerve preservation.
In these cases, parotidectomy was planned prior to surgery to exclude pleomorphic adenoma due to inconclusive results from FNA. In the first case, total parotidectomy was performed after intraoperative findings indicated invasion of the deep lobe, while in the second case, superficial parotidectomy was chosen considering the size and location of the mass. Both cases confirmed the pathology of BCA, specifically excluding the membranous type with its high recurrence rate. Furthermore, there were no permanent sequelae resulting from facial nerve injury or the surgery itself. With respective follow-up periods of 2 years and 3 months, there have been no instances of recurrence or complications. The indication for parotidectomy should be determined by considering the possibility of malignancy and the recurrence rate. It cannot be ignored if there is a diagnosis that indicates the need for parotidectomy based on FNA.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Ethical approval
Given the nature of the study and the absence of any potential risks to participants, this report got the IRB review exemption number from institution’s research ethics committee (KC21ZASE0682).
Patient consent
The patients provided written informed consent for publication and use of their images.
Author contributions
Conceptualization: Suk-Ho Moon. Methodology: Suk-Ho Moon. Writing - original draft: Sungyeon Yoon. Writing - review & editing: Sungyeon Yoon, Yesol Kim. Supervision: Suk-Ho Moon.
Abbreviations
BCA
basal cell adenoma
BCAC
basal cell adenocarcinoma
CT
computed tomography
FNA
fine needle aspiration
MRI
magnetic resonance imaging