Predicting recurrence in oral cavity cancers: a review of 116 patients with buccal mucosa carcinoma in northwestern India
Article information
Abstract
Background
Oral cavity cancers, the second most common type in India, are responsible for 10% of the overall cancer burden. With a recurrence rate of 30% to 40% and a 5-year survival rate of 50%, these malignancies account for substantial morbidity and mortality. Despite advances in treatment modalities, survival rates following treatment completion have not improved significantly. The present study aimed to establish specific epidemiological and pathological factors responsible for recurrence after treatment completion in buccal mucosa cancers.
Methods
A retrospective analysis of the data of 116 patients treated for biopsy-proven cancers of the buccal mucosa was undertaken 1 year after treatment completion. Factors such as age, sex, education, lymphovascular invasion, extranodal extension (ENE), perineural invasion, depth of invasion, and pathological margin status were compared between patients who presented with recurrence and those who did not. Statistical significance was set at p< 0.05.
Results
Of the 116 patients, 40 (34.5%) developed a recurrent disease within 1 year. The mean age of the study population was 43.3 years, and males constituted 91.4% of the included patients. Ipsilateral buccal mucosa was the commonest site of disease recurrence. Neck node metastasis, ENE, and margins of resection < 5 mm were significantly related to the recurrence of disease. However, surprisingly, lymphovascular invasion, perineural invasion, and depth of invasion > 10 mm did not show statistically significant associations.
Conclusion
Neck node metastasis, ENE, and margins of resection < 5 mm were the histopathological factors associated with recurrence in cancers of the buccal mucosa.
INTRODUCTION
Lip and oral cavity cancers pose an enormous burden on the Indian healthcare system. Accounting for 10.3% of the overall cancer cases in India, these malignancies are the second most common cancers diagnosed after breast malignancies and the third most common cause of cancer-related mortality after cancers of the breast and cervix [1].
With a cumulative 5-year survival rate of around 50%, which decreases to 30% in advanced presentations, these cancers are responsible for substantial morbidity and mortality [2]. Advances in treatment modalities, including the use of adjuvant chemoradiotherapy have not led to a significant improvement in survival in oral squamous cell carcinoma (OSCC) [3]. Locoregional recurrence (LRR) is seen in 10% to 30% of patients following treatment completion and is generally considered to be a predictor of an unfavorable prognosis regarding survival [4,5]. Despite the massive impact of recurrence on OSCC prognosis, we have only a limited understanding of the patterns and factors responsible for recurrence [3,6]. This can be attributed to the diverse nature of studies done previously, varying treatment strategies, and the fact that squamous cell carcinomas (SCCs) arising in different subsites of the oral cavity are distinct entities. The absence of specific biomarkers to predict each patient’s disease burden not only hinders our provision of effective treatment plans, but also leads to deficiencies in monitoring for recurrence [7].
The present retrospective observational study aimed to analyze the epidemiological and clinicopathological features associated with LRR in OSCC of the buccal mucosa (BM).
METHODS
Study design and place
The present study was conducted in the Department of Surgical Oncology, Sawai Man Singh Medical College, Jaipur, Rajasthan, India after clearance was obtained from the institutional ethics committee. Reports of 116 biopsy-proven cases of OSCC of BM diagnosed and treated between January 2020 and June 2021 were analyzed retrospectively. Data was collected and analyzed from the medical records department and outpatient documentation 1 year after completion of treatment.
The inclusion criteria for this study were as follows: (1) patients with biopsy-proven OSCC of BM; (2) patients aged >18 years; or (3) patients who completed treatment according to institutional protocols. Exclusion criteria for patients were as follows: (1) patients with SCC of another site of the oral cavity, such as the tongue or hard/soft palate; (2) patients presenting with a recurrence or distant metastasis; (3) patients who received a form of anti-cancer therapy previously, including surgery, chemotherapy, or radiotherapy; (4) those with positive surgical margins; or (5) those who were lost to follow-up or who did not complete treatment according to institutional protocols.
Treatment
All patients with biopsy-proven OSCC of BM were staged using contrast-enhanced computed tomography (CECT) scans of the face and neck, as well as a CECT chest scan to detect lung metastasis. All patients underwent a wide local excision of the primary carcinoma, including a segmental/marginal mandibulectomy (if needed) with neck dissection from levels 1 to 5 according to institutional protocols. Reconstruction was done using a pectoralis major myocutaneous flap, nasolabial flap, or free flap. The pathological stage was assigned postoperatively according to the eighth edition of the American Joint Committee on Cancer (AJCC) TNM classification [8]. Patients with positive neck node metastasis, T3/T4 stage disease, perineural invasion (PNI), depth of invasion (DOI) >5 mm, and closest margin of resection <5 mm received adjuvant treatment in the form of postoperative radiotherapy at a dose of 60 Gy to the tumor bed and ipsilateral neck. Those with extranodal extension (ENE) received additional chemotherapy in the form of carboplatin and paclitaxel for six cycles. Patients with positive margins were excluded from the study.
Follow-up
Follow-up occurred three times at 1-month intervals. A thorough physical examination was conducted along with a chest X-ray. Patients presenting with suspicion of recurrence underwent a CECT scan of the face and neck along with a CECT scan of the chest. Suspicious lesions were biopsied.
Statistical analysis
We investigated the following factors: age, sex, pathological stage, tumor (T) stage, nodal (N) stage, DOI, PNI, lymphovascular invasion (LVI), ENE, and closest margin of resection. The relationship of these factors with the recurrence of OSCC was established by comparing the data of those who had recurrences and those who did not. Statistical analysis was undertaken using SPSS version 23.0 (IBM Corp.). The prognostic significance of DOI and pathological margins was calculated at various cutoff values. Univariate and multivariate analyses were used to identify the independent risk factors for LRR. The chi-square test was used for the univariate analysis, and multivariate analysis of the prognostic factors was performed using the Cox logistic regression method. In all analyses, p<0.05 was considered significant.
RESULTS
The present study included 116 patients who underwent wide local excision and neck dissection, followed by reconstruction using a pedicled flap or a free flap, to manage OSCC of BM between January 2020 and June 2021. Adjuvant therapy in the form of radiotherapy or chemoradiotherapy was administered if indicated. Analyses took place 1 year after the completion of treatment. Forty patients (34.5%) exhibited recurrence and 76 patients (65.5%) were disease-free at 1 year after treatment completion. Among the 40 patients who developed recurrence, 25 (62.5%) were diagnosed within the first 6 months of treatment completion, and the other 15 cases (37.5%) were detected at the 1-year follow-up.
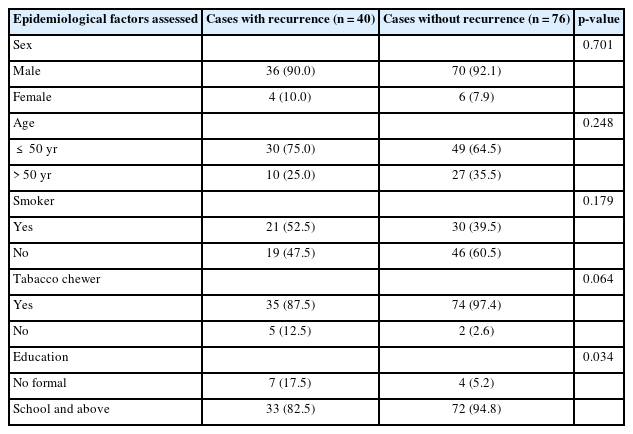
Males constituted 91.4% of the study population, with 106 cases, whereas females were affected in 10 cases (8.7%). Of the 116 patients, 79 (68.1%) were younger than 50 years, and 37 patients (31.9%) were aged 50 years or older at the time of primary presentation. Compared to the 109 patients (93.96%) who admitted to a regular tobacco chewing habit, only 51 patients (43.96%) admitted to a regular habit of smoking cigarettes or bidis. Although only a small number of patients (n=11, 9.5%) had not received any formal education, we found a statistically significant relationship (p=0.034) between the absence of formal education and development of LRR (Table 1).
The most common site of disease recurrence was the ipsilateral BM with 20 cases (50%). The ipsilateral neck was affected in 10 cases (25%), and the tongue was the site of recurrence in four cases (10%). In three patients (7.5%), recurrences developed on the pectoralis major myocutaneous flap used to reconstruct the defect. Contralateral BM was affected in three cases (7.5%).
On histopathological analysis, 99 patients (85.3%) presented with a moderately differentiated carcinoma, the commonest variety found in both the recurrence and non-recurrence groups. Interestingly, most patients in the recurrence group presented with a T2 disease (18 cases, 45%), followed by a T3 disease (17 cases, 42.5%). T4 disease was seen in only five patients (12.5%) in the recurrence group. In the group of patients presenting without recurrence, T4 was the commonest presentation (35 patients, 46.1%), followed by T3 disease with 20 patients (26.3%) (Table 2).
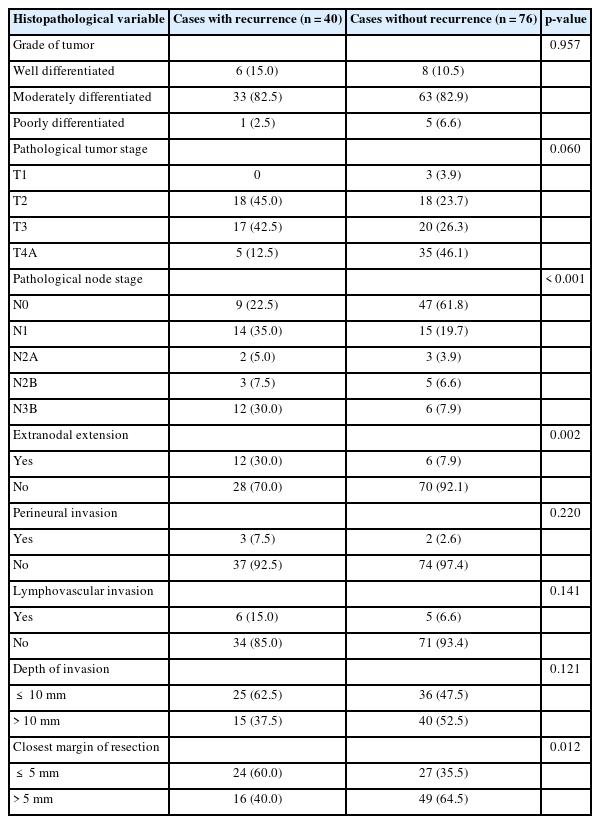
Comparison of histopathological features between patients with and without recurrence of oral cavity cancers
The present study found a significant correlation between neck node metastasis and development of LRR (p<0.001). N1 disease in 14 patients (35%) formed the commonest nodal presentation in the recurrence group; it is important to note that 12 patients (30%) in the recurrence group presented with ENE. In the group without recurrence, a pathologically uninvolved neck (N0) was the commonest presentation, with 47 cases (61.8%). Only six patients (7.9%) in this group had ENE, which also had a statistically significant impact on development of LRR (p=0.002) (Table 2).
Among the 116 cases included in our study, PNI and LVI were seen in only five cases (4.3%) and 11 cases (9.5%), respectively. Although higher rates of PNI and LVI were reported in the recurrence group, a statistically significant association between LRR and either PNI or LVI could not be determined (p>0.05).
To our surprise, patients in the non-recurrence group had more tumors with a deeper tumor penetration than those in the recurrence group. Forty (52.5%) of the 76 patients who did not develop a recurrence had a DOI >10 mm, whereas only 15 patients (37.5%) of the 40 patients reporting recurrence had a DOI >10 mm. The present study could not establish a statistically significant relationship between a larger DOI and LRR.
A significant association was seen between development of LRR and margins of resection that were less than 5 mm. Of the 40 patients presenting with recurrence, 24 patients (60%) had their closest margin of resection less than 5 mm, in contrast to only 27 patients (35.5%) in the non-recurrence group. The relationship between recurrence and the closest margin of resection being less than 5 mm was statistically significant (p=0.012) (Table 2).
DISCUSSION
Limited awareness of OSCC as a disease with substantial morbidity and mortality leads to patients visiting oncology clinics only when a growth inside the mouth fails to regress with time or is substantially painful.
Although 1 year of follow-up is a relatively short time to comment on recurrences in oral cavity cancers, as a tertiary care center in India, we deal mainly with advanced cases. Most of our patients who experience recurrence present within a year of treatment completion. Therefore, this analysis was performed at 1 year.
Men were predominantly affected in our study, accounting for 91.4% (106 cases) of the study population. Other reports from our region and other areas in the Indian subcontinent supported this trend of higher prevalence of OSCC in males [9-11]. Although the majority of patients in the Western world with oral cavity cancers present at an older age [12,13], the mean age of patients in our study was 43.3 years. Overall, 68% of our study population was younger than 50 years of age. Other studies from in and around India also reported a trend of younger patients presenting with oral cavity cancers [9,10]. Although two studies from Iran found a statistically significant relationship among a younger age of disease presentation, LRR, and survival [14,15], the present study could not establish such a correlation. Younger age of disease presentation and higher prevalence of OSCC in males in our study can be attributed to the rampant tobacco chewing behavior of young males in our region and a possibility of continuation of the habit even after undergoing treatment for OSCC, which might contribute to recurrence [16]. Many studies from around the world have concluded that the risk of LRR increases with age [12,13]. Increased duration of exposure to tobacco and other carcinogens may support more presentations and an increased incidence of recurrence in older age [17].
We found a statistically significant relationship between the absence of formal education and the incidence of recurrence in our population. Although only 11 patients (9.48%) in our study had not received formal education, a significantly higher proportion of patients in the group with recurrence had not received formal education compared to the group without recurrence (17.5% vs. 5.2%, respectively). The educated patients may have been more concerned about their ailment and attended their follow-up visits consistently. They probably avoided tobacco in any form posttreatment. We could not find any other previous literature to corroborate or dispute this finding.
Our study could not establish significance regarding the impact of higher T-stages on LRR. To our surprise, a higher T-stage was reported in more patients without recurrence than in those whose cancers recurred. In a meta-analysis on margin size and disease recurrence, Anderson et al. [18] asserted that the recurrence rates were similar between T1/T2 and T3/T4 groups. Other studies also failed to demonstrate the importance of an advanced T-stage for LRR [11,19,20].
We found a significant association between neck node metastasis and the development of recurrence whose significance was maintained in the multivariate analysis. The impact of pathologically involved nodes on recurrence has been documented by several studies from around the world [19,21,22]. Kartini et al. [15] reported that patients in Indonesia with N2 disease had a 1.4-fold higher risk of mortality than N0 patients. Several researchers have also reported an association between LRR and ENE [22,23]. Other studies, including a 2015 study from the Memorial Sloan Kettering Cancer Center, stated that the spread of disease 1.7 mm beyond the nodal capsule was associated with lower odds of disease-free survival after treatment [24,25]. We found ENE to be significantly associated with recurrence in the univariate analysis, but not in the multivariate analysis.
While the impact of tumor differentiation on LRR has been documented by several researchers [26,27], neither our study nor several others have established a significant relationship between tumor differentiation and prognosis [11,20]. It is important to note that moderately differentiated tumors affected more than 80% of the patients in both the recurrence and nonrecurrence groups in our study.
The inclusion of DOI in the new AJCC staging system for oral cavity malignancies demonstrates its significance in determining the prognosis of these tumors [8]. Many reports have documented a worse chance of disease-free survival as the DOI increases, especially to more than 10 mm [21,22]. The present study could not establish a significant impact of a DOI of more than 10 mm on LRR. To our surprise, though only 37.5% of the patients who developed recurrence had a DOI >10 mm, 52.5% of the patients who did not develop recurrence within 1 year of treatment completion had a DOI >10 mm.
Our study also could not establish any significant impact of PNI and LVI on LRR. Several studies, including that of Abbas et al. in Karachi [11], successfully established the significance of these factors for LRR [19,22]. Interestingly, Marinelli et al. [13] reported that PNI and LVI had an impact on overall survival but could not establish their role in LRR. Marzouki et al. [20] determined that PNI had a significant impact on LRR, whereas LVI was not found to have a statistically significant relationship with LRR.
Our study’s small sample size and its follow-up period of only 1 year could be responsible for the inability to demonstrate the effects of PNI and LVI on LRR in oral cavity cancers.
Apart from pathologically proven neck node metastasis, the only factor identified in the multivariate analysis as having a statistically significant relationship with LRR was the closest margin of resection measuring less than 5 mm. The width of the margins is of importance because epithelial dysplasia has been found near the surgical margins and can be an important cause of recurrence in patients not receiving adjuvant therapy [18]. Anderson et al. [18] and Carrillo et al. [19] also concluded that margins less than 5 mm had a greater risk of LRR. A few studies have questioned the impact of margins on the overall prognosis [5,20]. Abbas et al. [11] observed that although a positive margin was not associated with a poorer outcome, close margins were associated with a higher incidence of recurrence. These varying results could probably be explained by the uneven distribution of tumor sites and the impact of adjuvant therapy in patients with close or positive margins. Despite receiving adjuvant radiation, patients with close margins in our study had a significant association with recurrence. Adjuvant therapy probably did not have much of an impact in preventing recurrences in patients with close margins <5 mm.
The limitations of this study include its retrospective design with a relatively small sample size. We were not able to document the continued tobacco/betel nut chewing and smoking habits of our patients posttreatment. Another factor that could have impacted the results and was not assessed was the interval between surgery and the start of adjuvant treatment.
The present retrospective study found that the histopathological factors responsible for recurrence in patients presenting with OSCC were neck node metastasis, ENE, and close margins of resection during the primary surgery. Important factors established in previously conducted studies, such as PNI, LVI, DOI >10 mm, and an advanced T-stage, failed to show statistically significant associations with LRR in our study. It is incumbent upon us to recognize factors responsible for and predictive of recurrence. Aggressive treatment with early recognition of locoregional and distant failures will go a long way in increasing survival rates for OSCC. A study involving a greater number of patients with a prolonged follow-up will help us determine which factors are not only responsible for LRR but relevant to overall survival.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Ethical approval
The study was approved by the Sawai Man Singh Medical College of Ethics Committee (No. 101/MC/2023) and performed in accordance with the principles of the Declaration of Helsinki. The informed consent was waived because this study design is a retrospective chart review.
Author contributions
Conceptualization: Pinakin Patel, Pranav Mohan Singhal, Kamal Kishor Lakhera. Data curation: Agil Babu. Formal analysis: Pinakin Patel, Pranav Mohan Singhal, Kamal Kishor Lakhera, Agil Babu, Suresh Singh, Naina Kumar Agarwal. Methodology: Pinakin Patel, Pranav Mohan Singhal, Aishwarya Chatterjee, Agil Babu, Shubhra Sharmas. Project administration: Pinakin Patel, Pranav Mohan Singhal, Suresh Singh, Naina Kumar Agarwal, Shubhra Sharmas. Visualization: Pranav Mohan Singhal. Writing - original draft: Pinakin Patel, Pranav Mohan Singhal, Kamal Kishor Lakhera, Bhoopendra Singh Gora. Writing - review & editing: Pinakin Patel, Pranav Mohan Singhal, Aishwarya Chatterjee. Investigation: Pinakin Patel, Pranav Mohan Singhal, Kamal Kishor Lakhera, Aishwarya Chatterjee, Agil Babu. Resources: Pinakin Patel, Pranav Mohan Singhal, Aishwarya Chatterjee, Suresh Singh, Bhoopendra Singh Gora, Shubhra Sharmas. Supervision: Pinakin Patel, Pranav Mohan Singhal, Kamal Kishor Lakhera, Suresh Singh, Bhoopendra Singh Gora. Validation: Pinakin Patel, Pranav Mohan Singhal, Kamal Kishor Lakhera, Shubhra Sharmas.
Abbreviations
AJCC
American Joint Committee on Cancer
BM
buccal mucosa
CECT
contrast-enhanced computed tomography
DOI
depth of invasion
ENE
extranodal extension
LRR
locoregional recurrence
LVI
lymphovascular invasion
OSCC
oral squamous cell carcinoma
PNI
perineural invasion
SCC
squamous cell carcinoma