 |
 |
- Search
| Arch Craniofac Surg > Volume 24(5); 2023 > Article |
|
Abstract
Background
Botulinum toxin is a neurotoxic substance with a wide range of uses, from the treatment of musculoskeletal spasms to antiaging regimens by improving wrinkles. Split-face studies in which drugs are injected in the right and left sides of the faces have been actively conducted in botulinum toxin studies. In this study, we aimed to investigate the reliability of a split-face study for determining the effectiveness of botulinum toxin based on eyebrow height and movement, and electromyography results.
Methods
Thirty-one women aged 35 to 55 years were included in the study. Eyebrow height was measured as the distance from the eyebrows to the upper eyelid margin on the primary gaze, and eyebrow movement was measured as the distance when the forehead was wrinkled for 5 seconds. A noninvasive method was used for electromyography of the frontalis muscles.
Results
No statistically significant differences in right and left eyebrow heights and movements, and electromyography findings (p= 0.256, p= 1.000, and p= 0.978, respectively) were found. Pearson correlation analysis showed that electromyography muscle activity is positively associated with eyebrow movement, respectively (p< 0.001).
The forehead is anatomically the upper third of the face [1]. The frontalis muscle, one of the bellies of the occipitofrontalis, is one of the forehead muscles [2]. This muscle functions to elevate the eyebrow and horizontal wrinkles of the forehead [3].
Facial aging is a dynamic process that includes all tissue layers such as facial fat, facial bones, ligaments, muscles, and skin. The incidence and progress of the aging process vary in speed and severity from person to person [4]. Deep skin wrinkles can visually indicate the aging of the forehead. To elevate the eyelid tissue or sagging eyebrows in excess, the eyebrow-eyelid complex usually rises compensatively, resulting in chronic contraction of the frontalis muscle and the formation of wrinkles [5].
To prevent skin aging, research on skin rejuvenation is being actively conducted. Both surgical and nonsurgical approaches have been used to prevent facial aging [6]. The use of botulinum neurotoxin or a variety of fillers is one of the representative methods of skin rejuvenation.
Botulinum toxin was approved for temporary improvement of glabellar lines and has been used widely for various aesthetic purposes [7].
There are currently only two serotypes of neurotoxin available. Various botulinum toxins have been developed and marketed, including Botox (onabotulinumtoxinA) and Dysport (abobotulinumtoxinA). In 2000, the Food and Drug Administration also approved Myobloc (rimabotulinumtoxinB). Xeomin (incobotulinumtoxinA) was introduced 2005 [8].
Double-blind, split-face studies are being actively conducted to analyze the effects of drugs [9,10]. The split-face study in which the drug is administered by dividing the forehead area of the test subject in half was conducted to directly compare the effects of facial rejuvenation at a certain time after botulinum toxin administration. In this study, we investigated whether the split-face study is a reliable method for botulinum toxin research by comparing right and left eyebrow heights and movements, and the electromyography (EMG) signal of the frontalis muscle. In addition, by comparing the data of the study subjects, we examined the relationship between eyebrow movement and EMG muscle activity, changes in the right and left eyebrow heights and movements, and EMG muscle activity with age.
This study was approved by the Institutional Review Board of Gachon University Gil Medical Center, Republic of Korea (IRB No. GCIRB 2014-360). All the subjects signed a consent form, and the study was conducted in accordance with the principles of the Declaration of Helsinki.
The subjects were 31 women between 35 and 55 years of age. The exclusion criteria were as follows: patients who had received botulinum toxin injection, treatment with fillers or laser resurfacing within 24 months, and treatment with hyaluronic acid or other fillers within 12 months, and those with scars or marked asymmetry in the forehead region.
We measured eyebrow height and eyebrow movement, and EMG signal. Eyebrow height was defined as the distance (mm) from the eyebrow to the upper eyelid margin at primary gaze and measured on both sides. Eyebrow movement was measured as the distance (mm) the eyebrows moved by grimacing the forehead for 5 seconds. EMG studies were conducted on the frontalis muscles by using the BioPak system (BioResearch, Inc.). Electrode skin areas were cleaned before electrode placement. The reference electrode was located on the chin and measurement electrodes were attached to the center of the frontalis muscle, which is in the middle between the hairline and eyebrow above the pupil. The subjects were asked the forehead muscle was contracted to raise the eyebrow as far as possible. A specialist of rehabilitation medicine conducted the EMG tests and measured the waveform of the EMG. The data points were the average value of the maximal amplitude of the surface EMG muscle activity that appeared when the subjects contracted the frontalis muscle three times at maximum voluntary contraction.
The paired t-test was used to compare the eyebrow height and eyebrow movement between the right and left and the EMG values of the right and left frontalis muscles. We used the Pearson correlation analysis to study the correlation between eyebrow movement and EMG muscle activity and the changes in right and left eyebrow heights, movements, and EMG value with age. All statistical analyses were performed using SPSS Version 20 (IBM Inc.). A value of p<0.05 indicated statistical significance.
The study was conducted in 31 women with ages ranging from 35 to 55 years. The mean age of the group was 42.94±5.51 years.
The mean eyebrow heights of the right and left eyes were 13.24 and 13.55 mm, respectively. No statistically significant differences were found in the right and left eyebrow heights (p=0.256) (Table 1).
The distance of the eyebrows was measured by frowning the forehead, and the mean distances on the right and left sides were 7.68 and 7.68 mm, respectively. No statistically significant differences were found in the right and left eyebrow movements (p=1.000) (Table 1).
The mean frontalis muscle EMG values on the right and left sides were 181.90 and 181.79, respectively. No statistically signif-icant difference was found between the two groups (p=0.978) (Table 1).
The correlation coefficient between age and eyebrow height was 0.078, but not statistically significant (p=0.560).
By comparing eyebrow movement according to age, we found that the correlation coefficient is –0.032, but the correlation was not statistically significant (p=0.814).
The results of the EMG waveform with age were as follows: Analysis did not show any association with a correlation coefficient of 0.000 and was not statistically significant (p=0.999).
The EMG value had a weak positive linear association with the movement of the eyebrow with a correlation coefficient of 0.366 and was statistically significant (p<0.001) (Fig. 1).
Commercially available botulinum toxin formulations for treatment of dystonia in the United States include onabotulinumtoxinA (OnaBoNT-A), abobotulinumtoxinA (AboBoNT-A), incobotulinumtoxinA (IncoBoNT-A) and rimabotulinumtoxinB (RimaBoNT-B). Several botulinum neurotoxin formulations, including prabotulinumtoxina A-xvfs, daxibotulinumtoxin A (DaxiBoNT-A), neu-botulinumtoxinA (NeuBoNT-A), letibotulinumtoxin A, botulinum toxin E (rBoNT-E), Innotox, and QM-1114 are currently used in many countries, even though are not approved by Food and Drug Administration for the treatment of dystonia [11]. New botulinum toxins are continuously being developed, and split studies are being conducted to compare them with existing products. Botulinum toxin exists in seven types of neurotoxins (types A–G) [12]. A split-face study is being conducted to compare the effects of each type of botulinum toxin [13,14].
Meanwhile, the reliability of split-face studies of botulinum toxin is controversial. Studies have been conducted to address the unwanted side effects of the diffusion of botulinum toxin. Animal electrophysiological studies have shown that non-target muscles are affected by botulinum toxin injection. According to a study by Yaraskavitch et al. [15], when measuring force production and the muscle weakness of the target and adjacent non-target muscles after botulinum toxin injection, both muscle groups exhibited decreases regardless of the target. The result indicated that botulinum toxin can pass through the muscle fascia and reach adjacent muscles, causing muscle weakness [16]. The spread of botulinum toxin tends to be caused by the diffusion of unbound toxins through the extracellular space. This is caused by the concentration gradient and dynamics of the injection [17].
Measurements of the compound muscle action potential (CMAP), an indicator of efficacy and diffusion, showed a reduction not only in the ipsilateral but also in the contralateral glabellar muscles when on botulinum toxin A was injected in the corrugator muscle unilaterally [18]. In Girlanda et al. [19], single-fiber EMG signals and CMAP were measured in the orbicularis oculi muscles when botulinum toxin was injected in one eye and saline solution was injected in the other eye. As a result, facial CMAP decreased bilaterally, and the single-fiber EMG signal changed statistically significantly on both sides [19]. Therefore, the dilution and injection capacity must be adjusted because the injected botulinum toxin can spread and affect the opposite area.
Weise et al. [20] suggest that botulinum toxins not only affects neuromuscular junctions but may also have central effects on the central nervous system. In addition, botulinum toxin may affect antagonist muscles and other noninjected muscles in addition to the corresponding muscles. According to Matak [21], an axonally transported botulinum toxin mitigates muscle hypertonia caused by tetanus toxin. This process is attributed to the transsynaptic movement of botulinum toxin in the central nervous system.
We used surface EMG to measure the electrical activity of muscles from the body surface. Surface EMG, which is used by attaching an electrode to the skin surface, has the advantage of being able to measure temporary high-resolution muscle electrical activity and detect quickly and in a short period [22]. The main advantage of surface EMG is that it is noninvasive and painless, unlike using a needle. This method is also used in facial studies and diagnosis of facial myopathy or neuropathy because the EMG signal constantly changes with muscle movement [23-25].
In this study, we showed no significant differences in eyebrow height and movement, and EMG muscle activity on the right and left sides. Based on the comparison of data between study participants, no significant relationship was found between age and each indicator, but a significant result was obtained between eyebrow movement and EMG muscle activity. The EMG signal was positively associated with eyebrow movement by Pearson correlation analysis.
In conclusion, we demonstrated the reliability of the split study by finding no difference in eyebrow height or movement between the left and right sides. We also found a positive correlation between EMG of the frontalis muscle and eyebrow movement, proving the usefulness of EMG in rejuvenation research.
Notes
Ethical approval
The study was approved by the Institutional Review Board of Gil Medical Center (IRB No. GDIRB2014-360) and performed in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained from the patients.
Author contributions
Conceptualization: Yu Jin Kim. Data curation: Yun Sang Kim. Formal analysis: Hye Gwang Mun. Project administration: Yu Jin Kim. Visualization: Ki Young Park. Writing - original draft: Ki Young Park. Writing - review & editing: Bomin Moon. Investigation: Bomin Moon. Software: Hye Gwang Mun. Supervision: Yu Jin Kim.
Fig. 1.
Relationship between eyebrow movement and electromyography (EMG). The Pearson correlation analysis shows EMG value was weakly positively associated with eyebrow movement (Pearson correlation coefficient=0.366, p<0.001).
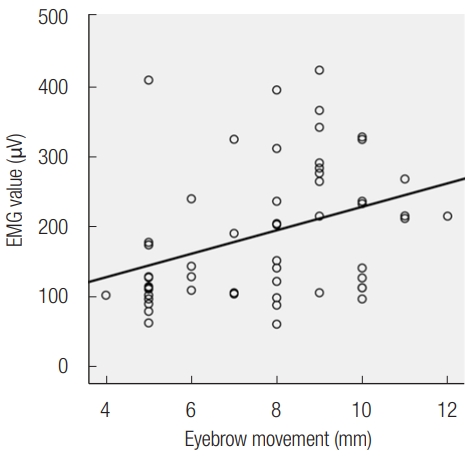
Table 1.
Eyebrow height, movement, and electromyography muscle activity (n=31)
|
Eyebrow, mean ± SD |
p-valuea) | ||
|---|---|---|---|
| Right | Left | ||
| Eyebrow height (mm) | 13.24 ± 2.61 | 13.55 ± 2.69 | 0.256 |
| Eyebrow movement (mm) | 7.68 ± 2.31 | 7.68 ± 1.92 | 1.000 |
| Electromyography muscle activity | 181.90 ± 98.56 | 181.79 ± 90.08 | 0.978 |
REFERENCES
1. Garritano FG, Quatela VC. Surgical anatomy of the upper face and forehead. Facial Plast Surg 2018;34:109-13.


2. Costin BR, Plesec TP, Sakolsatayadorn N, Rubinstein TJ, Mc-Bride JM, Perry JD. Anatomy and histology of the frontalis muscle. Ophthalmic Plast Reconstr Surg 2015;31:66-72.


3. Wieder JM, Moy RL. Understanding botulinum toxin: surgical anatomy of the frown, forehead, and periocular region. Dermatol Surg 1998;24:1172-4.

4. Cotofana S, Mian A, Sykes JM, Redka-Swoboda W, Ladinger A, Pavicic T, et al. An update on the anatomy of the forehead compartments. Plast Reconstr Surg 2017;139:864e-872e.


5. Langsdon P, Petersen D. Management of the aging forehead and brow. Facial Plast Surg 2014;30:422-30.


6. Arnaoutakis D, Bassichis B. Surgical and nonsurgical techniques in forehead rejuvenation. Facial Plast Surg 2018;34:466-73.


7. Park MY, Ahn KY. Scientific review of the aesthetic uses of botulinum toxin type A. Arch Craniofac Surg 2021;22:1-10.




8. Peng Chen Z, Morris JG Jr, Rodriguez RL, Wagle Shukla A, Tapia-Nunez J, Okun MS. Emerging opportunities for serotypes of botulinum neurotoxins. Toxins (Basel) 2012;4:1196-222.



9. Prager W, Wissmuller E, Kollhorst B, Williams S, Zschocke I. Comparison of two botulinum toxin type A preparations for treating crow’s feet: a split-face, double-blind, proof-of-concept study. Dermatol Surg 2010;36 Suppl 4:2155-60.


10. Karsai S, Adrian R, Hammes S, Thimm J, Raulin C. A randomized double-blind study of the effect of botox and dysport/reloxin on forehead wrinkles and electromyographic activity. Arch Dermatol 2007;143:1447-62.

11. Spiegel LL, Ostrem JL, Bledsoe IO. FDA approvals and consensus guidelines for botulinum toxins in the treatment of dystonia. Toxins (Basel) 2020;12:332.



12. Cocco A, Albanese A. Recent developments in clinical trials of botulinum neurotoxins. Toxicon 2018;147:77-83.


13. Shome D, Kapoor R, Khare S. Two different types of botulinum toxins: is there a difference in efficacy and longevity? J Cosmet Dermatol 2019;18:1635-41.



14. Choi JW, Youn CS, An HT, Yoo JY, Na JI, Park KC, et al. Combined use of botulinum toxin type A and B for forehead rhytides: a randomized, double-blind, split-face study. J Dermatolog Treat 2013;24:126-32.


15. Yaraskavitch M, Leonard T, Herzog W. Botox produces functional weakness in non-injected muscles adjacent to the target muscle. J Biomech 2008;41:897-902.


16. Shaari CM, George E, Wu BL, Biller HF, Sanders I. Quantifying the spread of botulinum toxin through muscle fascia. Laryngoscope 1991;101:960-4.


17. Ramirez-Castaneda J, Jankovic J, Comella C, Dashtipour K, Fernandez HH, Mari Z. Diffusion, spread, and migration of botulinum toxin. Mov Disord 2013;28:1775-83.



18. Punga AR, Eriksson A, Alimohammadi M. Regional diffusion of botulinum toxin in facial muscles: a randomised double-blind study and a consideration for clinical studies with split-face design. Acta Derm Venereol 2015;95:948-51.


19. Girlanda P, Quartarone A, Sinicropi S, Nicolosi C, Messina C. Unilateral injection of botulinum toxin in blepharospasm: single fiber electromyography and blink reflex study. Mov Disord 1996;11:27-31.


20. Weise D, Weise CM, Naumann M. Central effects of botulinum neurotoxin: evidence from human studies. Toxins (Basel) 2019;11:21.



21. Matak I. Evidence for central antispastic effect of botulinum toxin type A. Br J Pharmacol 2020;177:65-76.

22. Rantanen V, Ilves M, Vehkaoja A, Kontunen A, Lylykangas J, Makela E, et al. A survey on the feasibility of surface EMG in facial pacing. Annu Int Conf IEEE Eng Med Biol Soc 2016;2016:1688-91.


23. De Letter M, Vanhoutte S, Aerts A, Santens P, Vermeersch H, Roche N, et al. Facial nerve regeneration after facial allotransplantation: a longitudinal clinical and electromyographic follow-up of lip movements during speech. J Plast Reconstr Aesthet Surg 2017;70:729-33.


- TOOLS
-
METRICS

-
- 0 Crossref
- Scopus
- 849 View
- 24 Download
- Related articles in ACFS
-
The Signficance of Intraoperative Radiograph in Treatment of Zygoma Fracture.2007 October;8(2)






