|
 |
- Search
| Arch Craniofac Surg > Volume 24(5); 2023 > Article |
|
Abstract
Fibrous dysplasia (FD) is a rare skeletal disorder characterized by abnormal fibro-osseous connective tissue replacing normal bone. Despite its benign behavior, craniofacial FD can cause morphological disfigurement, headache, and even blindness as a result of the produced mass effect. Surgical resection is recommended when the patient shows apparent clinical symptoms or aggravating facial asymmetry. Postoperative complications have been reported, such as hematoma, surgical site infection, abscess formation, resorption of the bone graft used for reconstruction, and recurrence. An aneurysmal bone cyst (ABC) is a rare benign bony lesion that can occur secondary to preexisting bone tumor. Secondary ABCs in craniofacial FD are extremely rare in the literature, accounting for less than 30, all of which are either case reports or series. We report an extremely rare case of symptomatic secondary ABC arising from craniofacial FD that had been misdiagnosed with abscess formation or recurrence and was surgically removed. Notably, 17 years elapsed between the primary surgery and the complication of secondary ABC. The patient underwent total removal of secondary ABC. After surgery, symptoms were relieved, with no recurrence observed during a 6-month follow-up.
Fibrous dysplasia (FD) is a rare congenital skeletal disorder characterized by abnormal fibrous-osseous connective tissue replacing normal bone. The lesions have been reported to account for 2.5% to 7.0% of all benign bone tumors, with an equal predilection for both sexes [1]. The diagnosis of craniofacial FD is based on clinical, radiographic, and histopathologic features. Clinically, most FDs are asymptomatic and are discovered incidentally on radiographs. Ninety percent of craniofacial FD present before the age of 5 years, and no new lesions are reported beyond the age of 10 [2,3]. However, FD can present with severe symptoms like weakness, localized pain, deformity, fractures, and compromised vision or hearing. FD presents in monostotic and polyostotic forms; the monostotic form affects one bone and accounts for approximately 70% to 85% of cases, while the polyostotic form affects several bones [1,3,4]. Additionally, patients with polyostotic forms FD may have McCune-Albright syndrome. A conservative approach with observation and close monitoring for monostotic craniofacial FD is regarded the optimal approach because the FD generally stops growing after puberty [3-6]. But if the indication is clear, surgical methods such as bone excision or shaving must be considered. Aneurysmal bone cyst (ABC) is a rare benign bony lesion that can occur primarily or secondarily. Many bony tumorous conditions can be the cause of secondary ABC. However, secondary ABCs in craniofacial FD are extremely rare and have specific clinical features. In this report, we introduce a problematic case of a secondary ABC after surgical bone removal in craniofacial FD. Successful clinical experience in managing this condition is highly valued, given its rarity and specificity.
A 9-year-old boy with no other specific medical or familial history presented with significant facial asymmetry and proptosis of the right eye. His parents had noticed the asymmetry since he was 6 years old. The patient had no symptom such as pain or visual deformity. A computed tomography (CT) scan was taken to reach an exact diagnosis. The CT scan revealed the characteristic findings of FD, including diffuse bone thickening with ground-glass opacity involving the right superior orbital rim, roof, and frontoparietal skull. In particular, the FD in the orbital rim was most severe and the orbital roof suffered extensively from disease (Fig. 1). Fortunately, the disease did not involve the skull base; however, the disfigurement was severe and got worse as the patient grew older. Also, there was the possibility of optic nerve compression or malignant change if the disease deteriorated. Therefore, dysplastic bone excision surgery was planned after sufficient time of explanation and consultation with the parents.
Under general anesthesia, a bicoronal incision was made, and a full-layer scalp flap was pulled back. To access the orbital roof, a square-shaped frontoparietal skull bone flap elevation was performed by the neurosurgery department team. After dissection, the dysplastic superior orbital roof was removed and reconstructed with split autologous rib bones fixed with screws (Fig. 2). After bone flap reinsertion and fixation, the wound was closed with the insertion of one hemovac drain. The patient had an uneventful postoperative course, and the result was aesthetically satisfying. The pathologic result which showed irregular trabeculae of woven bone was consistent with FD. In the postoperative 7-year follow-up CT scan, the reconstructed orbital roof was well stabilized. Around the orbital roof, remnant fibrotic tissue was slightly thickened compared to the previous CT scan. However, the patient had no symptoms at all, and asymmetry was not conspicuous. Therefore, follow-up was finished.
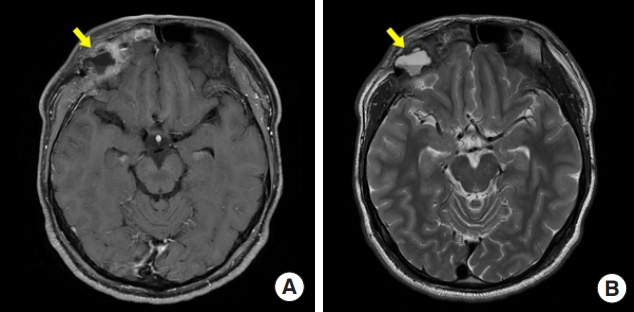
At the age of 26, the patient suddenly experienced a swollen feeling with sustained pain around the right superior orbital rim that was rated 3 on the numeric rating scale. Pain was aggravated by pressure. Blood laboratory tests, CT scan, and magnetic resonance imaging (MRI) were performed. Vital signs and laboratory results were all within the normal limits. The CT scan revealed a newly appearing 2.3 cm-sized low attenuation cystic lesion with adjacent fibrodysplastic bone at the orbital rim. The new lesion resorbed reconstructed the orbital roof (Fig. 3). In MRI, central diffusion restriction, T2 high signal, inner fluid-fluid level, and peripheral enhancement of the lesion were shown (Fig. 4). A local abscess was the primary differential diagnosis, and a tumorous condition was suspected but was less likely. The patient was hospitalized to receive intravenous antibiotics, and an exploration surgery was promptly planned.
The operation was performed under general anesthesia via a bicoronal incision along with the previous scar. Accessing the orbital roof was performed by neurosurgeons using the same method as that of the primary surgery. At the orbital rim, there was a very thin shell of FD. After removing the shell, we encountered the lesion. There was a soft tissue mass that grossly looked like granulomatous tissue with a brown color. A small amount of bleeding was found when the capsular structure surrounding the mass was opened. After excising the mass, shaving of dysplastic bone around the lesion and previous metal screw and plate removal were also performed. As seen from the CT scan and MRI, the anterior part of the reconstructed orbital roof was resorbed. Therefore, the parietal skull bone flap was harvested and split for orbital roof reconstruction. The outer cortex flap was reinserted in situ, and the inner cortical bone was carved and used for orbital roof reconstruction. Fixation was done with an absorbable screw (Fig. 5). The wound was closed with two hemovac drains.
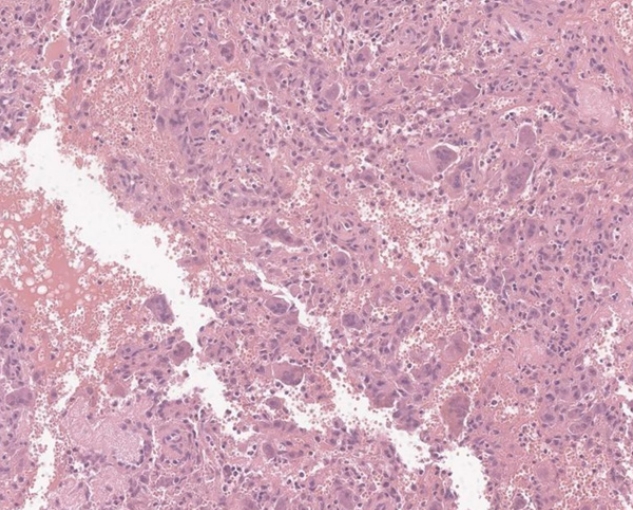
Pathologists reported that a multinucleated giant cell-rich lesion with irregular cystic space was shown, a finding that is consistent with an ABC (Fig. 6). There were no bacteria or fungi in the mass, as evidenced by microbiological culture. The patient’s symptom has been relieved after surgery with no postoperative complication or recurrence observed during the 6-month follow-up period.
Appropriate treatment of FD must be individualized and based on the patient’s specific presentation and chief complaint [7]. There are many variables, such as symptom, location, patient age, aesthetic expectation, etc. Especially in craniofacial monostotic FD, many surgeons do not prefer invasive surgery because monostotic FD have a relatively favorable prognosis. Patients usually have no symptoms, and disease progression after adolescence is minimal [1,3,4]. Resection of craniofacial bone can be destructive, so surgical risk may outweigh benefit in most cases. Many previous articles suggested indications of bone resection and immediate reconstruction. One absolute indication, which is widely accepted by many surgeons, is the patient having severe symptoms derived from the mass effect of dysplastic bone, like optic nerve compression. Another indication in asymptomatic cases, as suggested by one article, is craniofacial FD involving the fronto-orbital, zygomatic, mandibular, and/or hair-bearing cranium in an adult patient [8]. In adult patients, sarcomatous degeneration of FD has a 0.4% incidence, which about 400 times greater than in normal people [9].
In our patient, surgery is aimed at aesthetic improvement and reducing the potential for orbital nerve compression and malignant change. The patient and parents’ chief complaint was asymmetry of the face, especially the proptosis. The dysplastic orbital roof was removed and reconstructed with an autologous split rib bone graft. Reconstruction with autologous rib or clavicle was described in much of the previous literature. Bone grafts were valuable due to their low complication rates [10,11]. For reconstruction of the buttress or hard outer structure, titanium mesh was also widely used because of its hardness and relative ease to fabricate [12]. However, foreign bodies are naturally vulnerable to complications such as exposure or infection. The use of a three-dimensional printing rapid prototype model as a guide for the fabrication of autologous calvarial bone has recently resulted in satisfactory structural and aesthetic outcomes without any functional impairment or complications [13].
Recurrence of FD after bone resection is considered to be the result of remnant dysplastic bone due to imperfect resection. In craniofacial FD, recurrence after bone excision with reconstruction is rare [3]. There is one case report of recurrence after bone resection and reconstruction with titanium mesh. In that case, fibrodysplastic bone from resection margin seems like “soak” and “smear” the titanium mesh [14]. Although the reconstruction method was distinct from our case, the clinical features of recurrence were similar. In our first surgery, our team removed most of the dysplastic orbital roof. On gross inspection, the remnant bone was not seen to be dysplastic. Additionally, we used a rib bone that is totally non-dysplastic. Based on these factors, the risk of recurrence was thought to be low. However, recurrence occurred with an ABC on top. When dealing with the surgical approach to childhood FD, there are many difficulties derived from the uncertainty of the disease’s pathophysiology. Further basic research is required to ascertain the exact cause of the disease and its recurrence.
An ABC is a non-malignant bony lesion that consists of cystic cavernous cavities without lining endothelium filled with blood, commonly occurs in teenagers or young adults, and is rarely observed after the age of 30. ABC’s cause and pathogenesis are not certain, but the most commonly accepted hypothesis is that ABC is the consequence of increased venous pressure and the resultant dilatation and rupture of the local vascular networks [15]. ABC can occur either primarily or secondarily. Primary ABC is most often found in the metaphysis of long bones. Secondary ABC may arise within a preexisting bone tumor because of the changes in hemodynamics caused by the abnormal bone.
FD can also cause secondary ABC [3]. However, reports of secondary ABC occurring in craniofacial FD are extremely rare. All previous literature consists of either a case report or case series, with a total number of cases that is less than 30. ABC can be complicated with both monostotic and polyostotic FD. The speed of growth is relevant to secondary lesions such as ABC. Patients usually present with a rapidly enlarging, soft, painful mass in the preexisting FD, for which the treatment is a curative resection of the ABC with additional resection of the adjacent part of FD [3].
Our patient had no pain or neurologic symptom derived from FD. However, when the patient suddenly experienced swollen pain and bone tenderness, an ABC was identified in the dysplastic bone. As mentioned above, the speed of growth is relevant to the formation of secondary ABC. Our patient’s disease was diagnosed when he was under 10 years old. A secondary ABC was identified 20 years after the primary diagnosis of FD and 17 years after the primary surgical bone resection. Even though the patient was in his mid-20s when the secondary ABC complicated matters, this unusual feature made the diagnosis much more difficult.
By conducting a CT scan and MRI, a cystic bone erosive lesion with an inner fluid-fluid level and peripheral enhancement was identified. Our team primarily suspected an infectious condition such as an abscess, with the probable source of infection being the metal plates and screws used in the primary surgery. Although a tumorous condition was also considered, it was deemed less likely. The patient’s symptoms, such as sudden pain and bone tenderness, were consistent with those of an inflammatory condition. However, vital signs and blood laboratory results remained within the normal range. An exact diagnosis was difficult to make because of ambiguous clinical signs and symptoms, and a nonspecific radiological finding [15]. In ABC, a bone-erosive cystic lesion that is geographic and has a sclerotic border was shown in plain X-ray and CT scans. MRI has similar sensitivity and slightly higher specificity. In MRI, fluid-fluid levels, perilesional edema, and septation were shown. In ABC, 96.7% of the disease had some form of contrast enhancement on MRI. Septal enhancement was much more common with ABC than with other bone lesions [16]. Cyst formation within lesions of FD is not always indicative of secondary changes; it can also be seen in the natural course of the disease with advancing age. It is crucial to differentiate secondary ABC formation from the cystic variant of FD. Secondary ABCs usually expand much more rapidly than FD [17]. In our case, disease progression occurred over a relatively long period of time. Other benign bone tumors such as giant cell tumor, fibrosarcoma and brown tumor with internal hemorrhage can also show fluid-fluid levels [18]. Especially in the craniofacial area, ABC’s radiological findings had some features similar to those of a mucocele [19]. A cautious approach must be taken while considering multiple differential diagnoses.
The rapid growth and bone erosion associated with ABC resulted in significant pain for the patient. We removed ABC and shaved the adjacent dysplastic bone. The pain was relieved immediately after the procedure. After a 6-month follow-up, there has been no recurrence. The reported recurrence rate for ABC ranges from 10% to 50% [20], although there are no reports about the recurrence of secondary ABCs only. In our case, the patient was 26 years old. FD will not deteriorate and the ABC may not recur. However, this case is complicated and has specific clinical characteristics that do not match with previous case reports. Close follow-up is mandatory.
We aim to share our experience with the challenges of diagnosing secondary ABC. While secondary ABC has distinct clinical features and imaging findings, its extreme rarity can lead to misdiagnosis. Through this case report, we hope to improve understanding and diagnosis of secondary ABC.
Notes
Ethical approval
The report was approved by the Institutional Review Board of Seoul National University Hospital (IRB No. H-2304-119-1425).
Patient consent
The patient provided written informed consent for the publication and use of his images.
Author contributions
Conceptualization: Seungchul Baek, Byung Jun Kim. Data curation: Seungchul Baek, Byung Jun Kim. Methodology: Byung Jun Kim. Project administration: Seungchul Baek. Writing - original draft: Seungchul Baek. Writing - review & editing: Seungchul Baek, Byung Jun Kim. Supervision: Byung Jun Kim.
Fig. 1.
Primary diagnosis of fibrous dysplasia based on computed tomography scan at patient’s 9-year-old. The orbital roof suffered extensively from fibrous dysplasia.
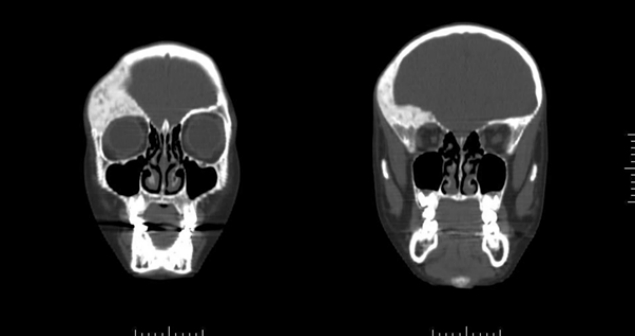
Fig. 2.
Intraoperative photographic finding from primary surgery. (A) After removal of dysplastic orbital roof. (B) Reconstructed orbital roof with split rib bone (black arrow: rib bone).
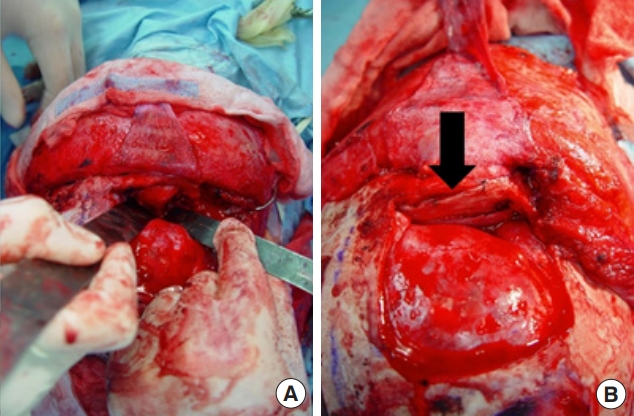
Fig. 3.
Computed tomography scan after symptom occur. Low-attenuation cystic lesion was newly appeared. It resorbs reconstructed orbital roof.
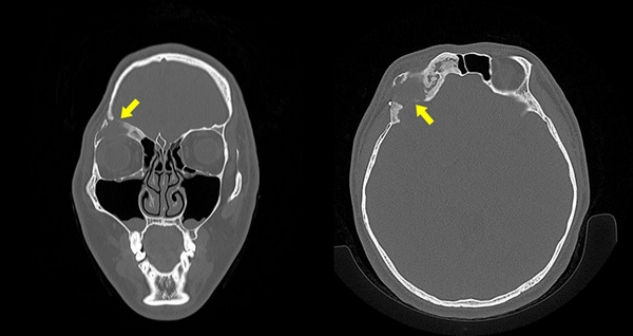
Fig. 4.
Magnetic resonance imaging after symptom occur. (A) Peripheral enhancement in T1 weighted after enhancement imaging. (B) Fluid-fluid collection in T2 weighted image which shows an airfluid level.
REFERENCES
1. DiCaprio MR, Enneking WF. Fibrous dysplasia: pathophysiology, evaluation, and treatment. J Bone Joint Surg Am 2005;87:1848-64.


2. Adetayo OA, Salcedo SE, Borad V, Richards SS, Workman AD, Ray AO. Fibrous dysplasia: an overview of disease process, indications for surgical management, and a case report. Eplasty 2015;15:e6.


3. Lee JS, FitzGibbon EJ, Chen YR, Kim HJ, Lustig LR, Akintoye SO, et al. Clinical guidelines for the management of craniofacial fibrous dysplasia. Orphanet J Rare Dis 2012;7 Suppl 1:S2.



4. Kim DY. Current concepts of craniofacial fibrous dysplasia: pathophysiology and treatment. Arch Craniofac Surg 2023;24:41-51.




5. Park JK, Lee SY, Kim JH, Kim BK. Long-term outcomes after core extirpation of fibrous dysplasia of the zygomaticomaxillary region. Arch Craniofac Surg 2023;24:59-65.




6. Kim GH, Yoon YS, Kim EK, Min KH. Frontal peripheral osteomas: a retrospective study. Arch Craniofac Surg 2023;24:24-7.




7. Panda NK, Parida PK, Sharma R, Jain A, Bapuraj JR. A clinicoradiologic analysis of symptomatic craniofacial fibro-osseous lesions. Otolaryngol Head Neck Surg 2007;136:928-33.



8. Denadai R, Raposo-Amaral CA, Marques FF, Ghizoni E, Buzzo CL, Raposo-Amaral CE. Strategies for the optimal individualized surgical management of craniofacial fibrous dysplasia. Ann Plast Surg 2016;77:195-200.


9. Feingold RS, Argamaso RV, Strauch B. Free fibula flap mandible reconstruction for oral obstruction secondary to giant fibrous dysplasia. Plast Reconstr Surg 1996;97:196-201.


10. Chen YR, Noordhoff MS. Treatment of craniomaxillofacial fibrous dysplasia: how early and how extensive? Plast Reconstr Surg 1990;86:835-44.

11. Valentini V, Cassoni A, Marianetti TM, Terenzi V, Fadda MT, Iannetti G. Craniomaxillofacial fibrous dysplasia: conservative treatment or radical surgery? A retrospective study on 68 patients. Plast Reconstr Surg 2009;123:653-60.


12. Pruksapong C, Lohwongwatana B. Orbitocranial fibrous dysplasia: immediate reconstruction with titanium 3-dimensional printing. Plast Reconstr Surg Glob Open 2020;8:e3114.



13. Ahn SJ, Hong JW, Kim YO, Lew DH, Lee WJ. Treatment of fibrous dysplasia of the zygomaticomaxillary complex with radical resection and three-dimensional reconstruction with autologous calvarial bone graft. Arch Craniofac Surg 2018;19:200-4.




14. Wu H, Li J, Xu J, You C, Huang S. Recurring craniofacial fibrous dysplasia with extensive titanium mesh invasion. J Craniofac Surg 2014;25:697-9.


15. Lin WC, Wu HT, Wei CJ, Chang CY. Aneurysmal bone cyst arising from fibrous dysplasia of the frontal bone (2004:2b). Eur Radiol 2004;14:930-2.



16. Restrepo R, Zahrah D, Pelaez L, Temple HT, Murakami JW. Update on aneurysmal bone cyst: pathophysiology, histology, imaging and treatment. Pediatr Radiol 2022;52:1601-14.




17. Kushchayeva YS, Kushchayev SV, Glushko TY, Tella SH, Teytelboym OM, Collins MT, et al. Fibrous dysplasia for radiologists: beyond ground glass bone matrix. Insights Imaging 2018;9:1035-56.




18. Davies AM, Evans N, Mangham DC, Grimer RJ. MR imaging of brown tumour with fluid-fluid levels: a report of three cases. Eur Radiol 2001;11:1445-9.