An intercontinental comparison of the influence of smoking on the occurrence of nonsyndromic cleft lip and palate: a meta-analysis and systematic review
Article information
Abstract
Background
The influence of smoking on nonsyndromic clefts has been a topic of research for many years. However, few studies have investigated the effect of smoking on causing clefts in different gene pools.
Methods
A meta-analysis was conducted of case-control studies related to smoking. Keywords such as “clefts,” “cleft lip,” “cleft palate,” “orofacial cleft,” and “smoking” were used to search the MEDLINE, Embase, and Cochrane databases.
Results
In total, 51 articles were reviewed. The RevMan software was utilized for the analysis, and the Mantel-Haenszel method was employed to pool the odds ratios (ORs) and 95% confidence intervals. Although the overall OR, a measure of the association between exposure and outcome, was higher for smokers than for non-smokers, this association was significantly stronger in individuals from Asia and South America (1.73), and lowest in Europe (1.31). Among active and passive smokers in Asia, the OR was approximately 0.93, indicating an equivalent impact from both types of smoking.
Conclusion
This analysis indirectly suggests that restriction measures targeting both active and passive smoking are crucial in Asia.
INTRODUCTION
Maternal smoking has various impacts on the fetus, but it is not always clear whether a mother has engaged in smoking or been exposed to tobacco. Some research has explored the significance of the dose-dependent effect of tobacco on cleft conditions, a category of severe congenital abnormalities. Although systematic reviews and meta-analyses have previously been conducted on this association, it is crucial to re-evaluate the data, incorporating all studies, including recent ones, to enhance our existing understanding. Given the multifactorial etiology of cleft conditions, this study aimed to conduct an intercontinental subgroup analysis of currently available data. Additionally, we aimed to investigate potential genetic influences on the occurrence of cleft conditions among mothers exposed to tobacco. The assumption underlying this review was that a study conducted on a specific continent was likely to primarily reflect the gene pool of that continent.
MATERIALS AND METHODS
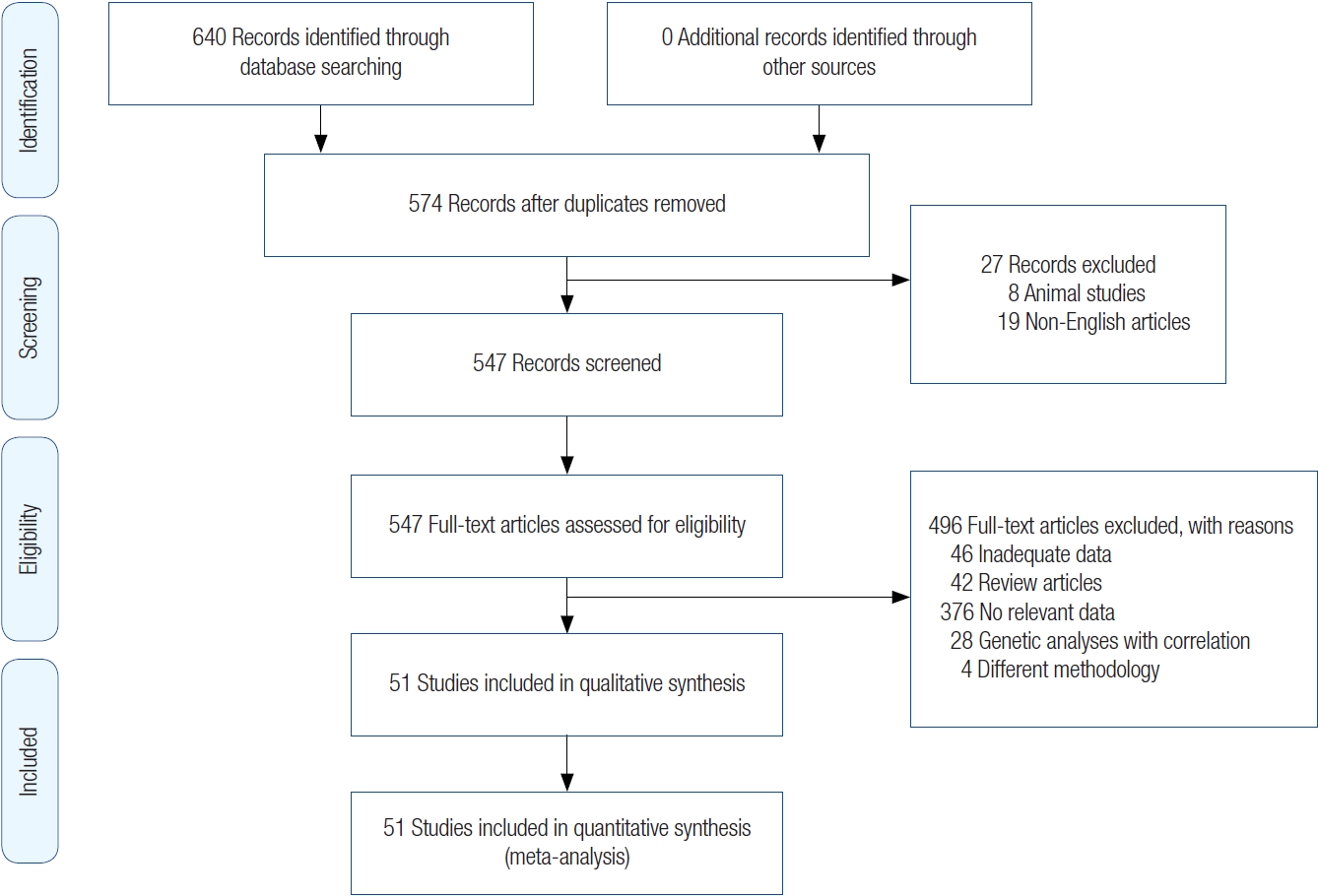
The search strategy was as follows: we searched for articles that discussed the impact of smoking prior to and during the first trimester of pregnancy on the occurrence of clefts (periconceptual period). Search terms such as “clefts,” “cleft lip,” “cleft palate,” “orofacial cleft,” and “smoking” were used to identify studies in the MEDLINE, Embase, and Cochrane databases from 1989 to November 21, 2021. We limited the search to case-control designs. Further refinement of the articles was done using the filters “human subjects” and “articles for which a complete English translation can be retrieved.” All the articles were catalogued in Excel spreadsheets, and a PRISM diagram is shown in Fig. 1.
The inclusion criteria were as follows: (1) studies that reported active and passive smoking, specifically focusing on women in the peri-conceptual period who are exposed to cigarette smoke by their partners or other close contacts, and analyzed the relationship between smoking (both active and passive) and nonsyndromic clefts through a comparison of the incidence in smokers and non-smokers; and (2) case-control studies that described both comparison and control groups.
The exclusion criteria were as follows: (1) studies that included smoking but did not specifically mention the association with clefts; (2) studies that included smoking but did not analyze the marginal effect on clefts, instead elaborating on the genetic modifications that led to clefts; or (3) studies that analyzed the association of smoking with syndromic clefts and studies from which data on nonsyndromic clefts could not be retrieved.
The following information was derived from the pooled data: (1) the prevalence of active and passive maternal smoking prior to and during the first trimester of pregnancy; (2) sample size, definitions of passive and active smoking, and base population; (3) study design; (4) study subtypes (and hence population subtypes) based on the continent where the study was conducted, presuming that a study from a particular continent would predominantly include a population originating from that continent; or (5) association between smoking and cleft lip and/or palate: odds ratios (ORs) and confidence intervals (CIs) without frequencies were included when available.
Quality of studies
The studies were categorized based on the Newcastle-Ottawa Scale into good, fair, and poor-quality studies. Those of poor quality were excluded from the analysis.
Risk of bias
The Newcastle-Ottawa Scale was employed to evaluate the risk of bias, as all the included studies were observational rather than randomized controlled trials. Further assessments were conducted using funnel plots and L’Abbé plots.
Small study effects
Funnel plots were utilized to visually represent the effects of small studies. This was done for all studies collectively, as well as for specific subsets, which dealt with the relationship between active and passive smoking with clefts, various types of clefts, and intercontinental studies.
Statistical analysis
We conducted a meta-analysis using the free software, ReviewManager (RevMan). The Mantel-Haenszel method was employed to combine studies and calculate summary ORs and 95% CIs for active or passive smoking. We tested for homogeneity and heterogeneity using the I2 statistic. A value exceeding 50% indicated moderate heterogeneity, while a value over 75% signified severe heterogeneity. For further analysis of the odds, we used a fixed-effect model for homogeneous studies and a random-effect model for heterogeneous studies. ORs, accompanied by 95% CIs, were graphically depicted in forest plots. Crude and adjusted ORs were calculated with 95% CIs. Further derivations were conducted using inverse variance weights and restricted maximum likelihood.
Subgroup analyses
Subgroup analyses were conducted according to the type of clefts, the type of smoking, and the continent from which the sample population was selected (i.e., the population pool).
RESULTS
After applying filters such as “case-control studies,” “human,” and “English language” to the MEDLINE database, 621 articles were retrieved. A search of the Embase database yielded 19 articles. A search of the Cochrane database did not produce any suitable articles. Therefore, a total of 640 records were identified through database searching. Out of them, a total of 574 articles were gathered for review after removing duplicates and applying the inclusion and exclusion criteria. Each article was meticulously examined for relevant data, resulting in a final selection of 51 articles [1-51]. The search process was carried out by two authors (MV, NS). In instances where there was disagreement about the inclusion or exclusion of a particular article, a third author (PK) was consulted for resolution.
Smokers versus non-smokers and cleft occurrence
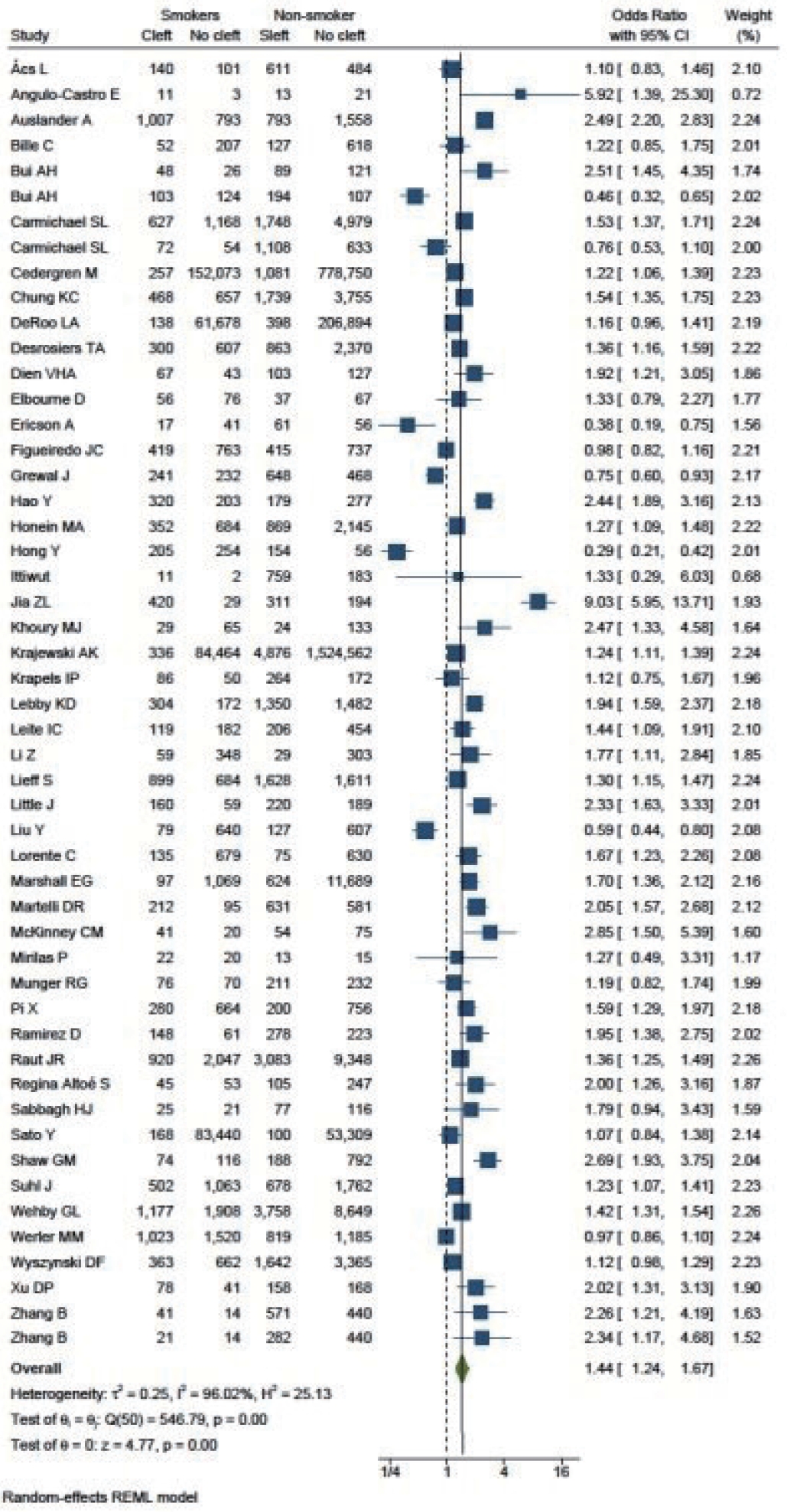
A total of 51 studies were included in the final analysis. The cumulative OR was 1.44 (95% CI, 1.24–1.67). The I2 test resulted in a value of 96.02%, indicating a high degree of heterogeneity among the studies, thus necessitating the application of a random-effect model. The value of τ2 was 0.25 (Fig. 2). Both funnel plots and L’Abbé plots were generated to assess the likelihood of publication bias.
Active versus passive smoking and cleft occurrence
We shortlisted six relevant studies that provided details on active and passive smokers in relation to cleft occurrence. The combined OR was 1.11 (95% CI, 0.87–1.41). The I2 value demonstrated homogeneity, as indicated by a value of 28.53% (Fig. 3).
Influence of the continent on clefts among smokers
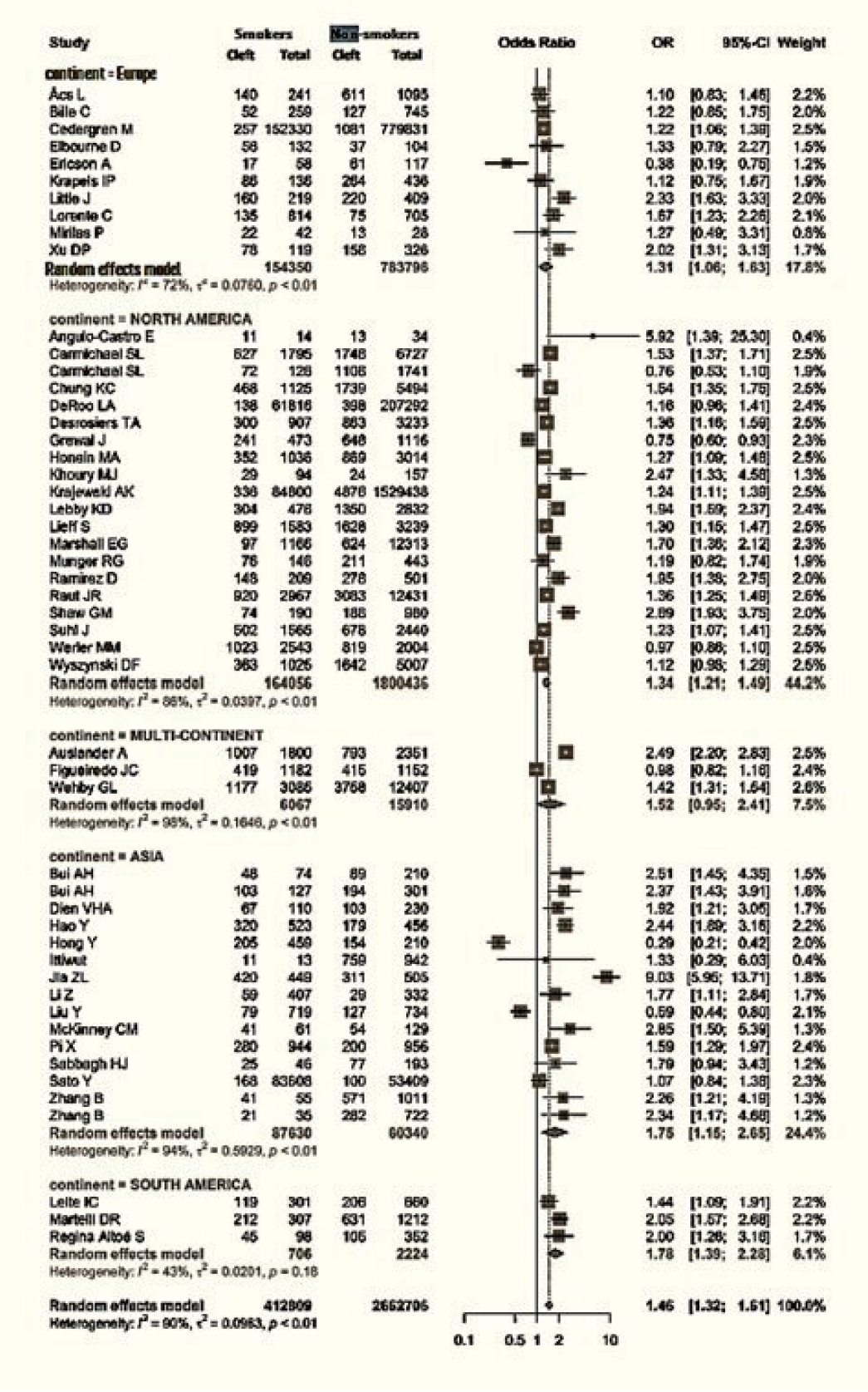
Data regarding the continent of origin were available for 51 studies. The studies were conducted in Asia (15 studies), Europe (10 studies), North America (20 studies), and South America (3 studies), and three studies spanned multiple continents. The cumulative OR from multicontinental data was 1.52 (95% CI, 0.95–2.41). For Asia, the OR was 1.75 (95% CI, 1.15–2.65). Europe had an OR of 1.31 (95% CI, 1.06–1.63), while that of North America was 1.34 (95% CI, 1.21−1.49). Lastly, South America had an OR of 1.78 (95% CI, 1.39−2.28) (Fig. 4).
Active versus passive smoking according to continent
The cumulative OR for Asia was 0.93, while the rest of the continents had ORs exceeding 1 (Fig. 5).
Smokers versus non-smokers and subgroups of clefts
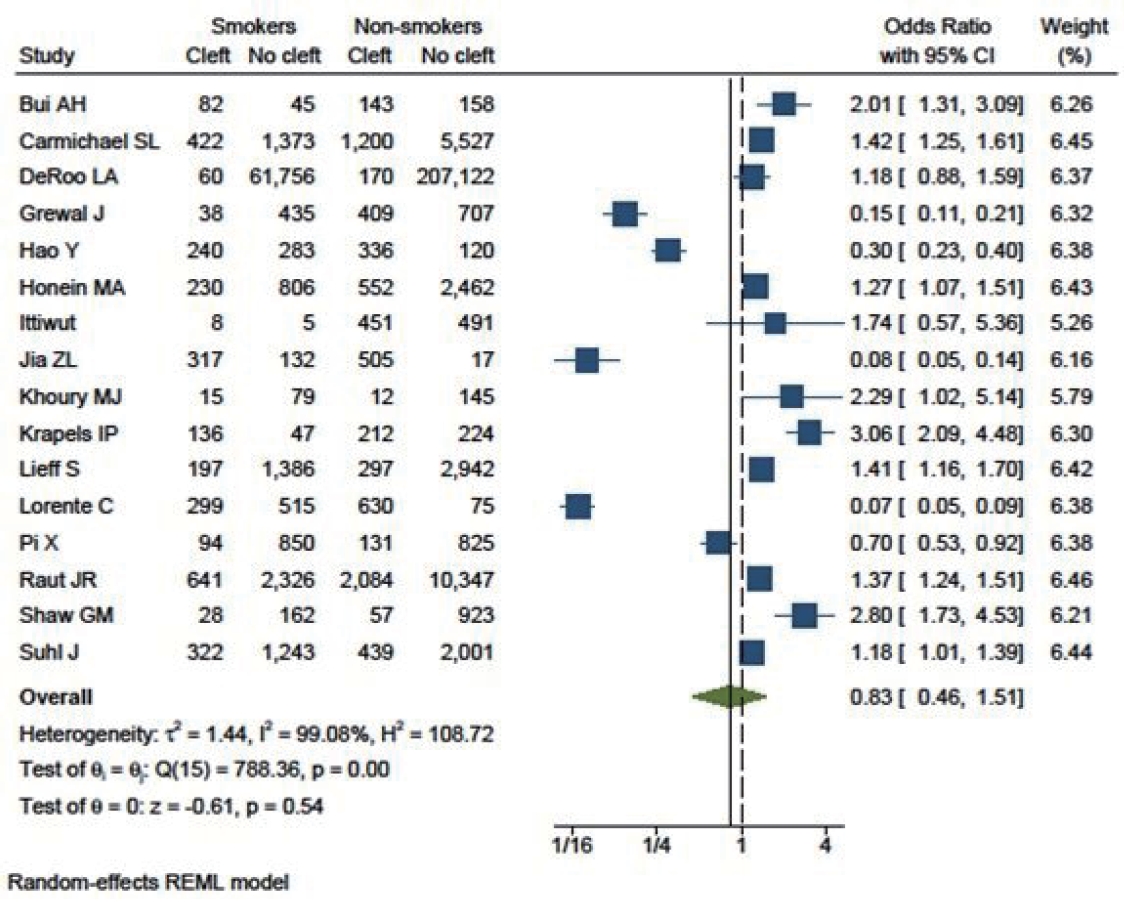
Three studies that included cleft lips yielded an OR of 1.71 (95% CI, 0.54–5.39). Eighteen studies specifically included cleft palate patients, with an OR of 0.89 (95% CI, 0.68–1.16). Additionally, 16 studies focused on patients with both cleft lip and palate (OR, 0.83; 95% CI, 0.46–1.51). This analysis indicated that smoking had a greater influence on cleft lip (as shown in Fig. 6) than on cleft palate (Fig. 7) or cleft lip with palate (Fig. 8).
Summary of results
Smokers vs. non-smokers
Figs. 1 and 2 demonstrate that children born to smokers (OR, 1.44; 95% CI, 1.24–1.67) were more likely to have cleft lip or cleft palate than children born to non-smokers. The study results exhibited a significantly high level of heterogeneity, with an I2 value of 96%. A subgroup analysis by continent did not reduce this heterogeneity. The association was stronger in studies reported from Asia and South America, with pooled ORs of 1.75 and 1.78, respectively. Conversely, studies from Europe presented the lowest OR of 1.31. The symmetry of the funnel plot suggests the absence of a small study effect in our analysis.
Active vs. passive smokers
Fig. 3 illustrates that active smokers exhibited 1.11 times higher odds of developing cleft lip or cleft palate than passive smokers (OR, 1.11; 95% CI, 0.87−1.41). However, this association was not statistically significant. The studies demonstrated low heterogeneity (I2= 29%).
Cleft lip only
Children born to smokers were found to have higher odds of developing cleft lip (OR, 1.71; 95% CI, 0.54–5.39); however, this association was not statistically significant. It should be noted, though, that only three studies reported this outcome, and moderate heterogeneity was observed among these studies.
Cleft palate only
The relationship between smoking and cleft palate was not found to be significant (OR, 0.89; 95% CI, 0.68–1.16). There was a significantly high level of heterogeneity among the studies (I2= 94%). The symmetry of the funnel plot suggests the absence of publication bias.
Cleft lip and palate
Children born to smokers were found to have a lower likelihood of developing cleft lip and palate than those born to non-smokers (OR, 0.83; 95% CI, 0.46–1.51). However, this association was not statistically significant. The study results exhibited high heterogeneity. The funnel plot was asymmetrical, with some studies reporting a highly significant protective effect of smoking.
DISCUSSION
Nonsyndromic clefts account for 70% of all reported cleft cases. Their etiology can be attributed to both genomic factors and epigenomic modifications. Genetic factors include MSX, IRF-6, FOX E, TGF-β, and others [52], and epigenomic influences from folate [53], smoking [54], and other factors have also been described.
Epigenetic factors such as cessation of smoking [55], vitamin supplement intake, calcium intake, and environmental pollution, play a crucial role in prevention. These factors are of particular importance because they are modifiable, unlike genetic factors.
Nicotine in tobacco is implicated in causing clefts through several mechanisms. Primarily, its vasoconstrictive effect can decrease uterine blood flow and oxygen delivery to the developing fetus, thereby disrupting both neural development and palatal fusion [56]. Additionally, nicotine may directly affect cleft palate by altering gene expression [57], which can lead to the persistence of epithelial cells in areas where connective tissue should fuse.
Thus, smoking cessation can be highly effective as a strategy to prevent clefts. A quantitative assessment of the association with clefts can provide caregivers and health workers with real-time data to emphasize when educating individuals about the benefits of quitting smoking. Although several systematic reviews and meta-analyses have explored this topic, their results have largely been inconsistent. These studies have typically focused on either active or passive smoking. Despite acknowledging the potential for heterogeneity bias in their research, these studies have not eliminated one of the most significant preventable causes of this issue—namely, the broad, non-specific genetic pool of the population studied. While smoking is a common etiological factor, different genetic pools may have varying susceptibilities to nicotine, and therefore, may not all have the same risk of developing clefts.
The proportion of active to passive smoking varies in different parts of the world [58]. For example, in Western countries, it is relatively common for women to smoke, unlike in certain Asian countries, where it is considered taboo for women to do so. Therefore, it is reasonable to anticipate that the susceptible female population in these countries, and consequently the continent, is more exposed to passive smoking than active smoking. While acknowledging the potential difficulty in eliminating genetic bias due to the dynamic population exchange among various countries, the authors of this study have nonetheless endeavored to further subanalyze the pooled data based on the continents of origin of the patients included in these studies. Nonetheless, a key assumption here is that the genetic pool included in a specific study corresponds to the same continent where the study was conducted.
Our analysis has demonstrated that smoking is a risk factor, as evidenced by the cumulative odds ratio of 1.44 for clefts, consistent with previous studies such as Crossan and Duane [59], which stated that the OR was 1.368 for cleft lip and/or cleft palate and 1.241 for cleft palate in actively smoking mothers. The high heterogeneity of the data could have been due to differences in the prevalence of smoking among different populations.
The results of a sub-analysis of the data by continent consistently indicated that smoking was a risk factor in all populations considered in the study, including those in Asia, Europe, and North and South America. Furthermore, the study demonstrated that active smoking poses a greater risk than passive smoking, with an OR of 1.11. This finding aligns with the conclusions drawn by Honein et al. [19].
However, the intercontinental analysis of active and passive smoking revealed no significant difference (with an OR of less than 1) in the Asian population compared to those in Europe, North, or South America (where the OR was more than 1). Despite the limited number of studies included in this analysis, there is essentially no evidence that would support dismissing the potential effects of passive smoking in causing clefts among populations in continents such as Asia.
The study conducted by Ma et al. [60] showed that there has been an increase in passive or secondhand smoke exposure in Asian countries. Despite smoking bans, compliance in middle and low-income countries (such as many of those in Asia) has not decreased as significantly as in other regions, due to political or economic factors. The impact of passive smoking on the occurrence of nonsyndromic clefts has been found to be greater than that of active smoking, as documented in the systematic review by Sabbagh et al. [61]. Since Asia is in the initial stages of the tobacco smoking epidemic [62], stricter restrictions need to be implemented on both active and passive smoking to prevent clefts, particularly in these regions.
A sub-analysis of the types of clefts identified smoking as a definitive risk factor for pure cleft lip, as compared to cleft palate or cleft lip with palate (with ORs of 1.71 versus 0.89 and 0.83). This finding contrasts with the study conducted by Kummet et al. [63], in which the OR was 1.16 for all subtypes of clefts.
CONCLUSION
This meta-analysis indicates that smoking is a risk factor for the development of clefts. It further demonstrates that the impact of both types of smoking is particularly detrimental to the Asian population, to a greater extent than for populations from Europe, North America, and South America.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Funding
This review was supported from Maastricht Educational and Research Centre Pvt Ltd.
Author contributions
Conceptualization: Madhubari Vathulya, Neetu Singh, Manisha Naithani, Peter Kessler. Data curation; Formal analysis; Funding acquisition: Madhubari Vathulya. Methodology: Madhubari Vathulya, Neetu Singh, Manisha Naithani. Project administration: Peter Kessler. Writing - original draft: Madhubari Vathulya. Resources; Software: Madhubari Vathulya. Supervision: Peter Kessler. Validation: Manisha Naithani.