 |
 |
- Search
| Arch Craniofac Surg > Volume 25(2); 2024 > Article |
|
Abstract
Because facial nerve injuries affect the quality of life, leaving them untreated can have devastating effects. The number of patients with traumatic and iatrogenic facial nerve paralysis is considerably high. Early detection and prompt treatment during the acute injury phase are crucial, and immediate surgical treatment should be considered when complete facial nerve injury is suspected. Symptom underestimation by patients and clinical misdiagnosis may delay surgical intervention, which may negatively affect outcomes and in some cases, impair the recovery of the injured facial nerve. Here, we report two cases of facial nerve injury that were treated with nerve grafts during the subacute phase. In both cases, subacute facial nerve grafting achieved significant improvements. These cases highlight surgical intervention in the subacute phase using nerve grafts as an appropriate treatment for facial nerve injuries.
Traumatic facial nerve injuries, including iatrogenic events, temporal bone fractures, and deep lacerations, are frequently observed in medical practice [1]. If not treated properly, facial nerve injuries may cause functional, aesthetic, and psychological problems. The best treatment for complete transection of the nerve is immediate, tension-free, end-to-end direct suturing. When a direct suturing is not possible, surgical alternatives can be considered based on the type and degree of injury, and the length of the gap between the nerve stumps [2-4]. Proper timing of nerve repairs can achieve better outcomes [4]. In cases where immediate repair with direct suturing is not feasible, delays beyond a critical time point can significantly limit the chances of successful surgical outcomes [2,3]. While the medical community continues to debate delayed nerve repair, multiple studies with promising results have been published. Here, we report two cases in which nerve grafting during the subacute phase of facial nerve injury achieved significant improvements and share our clinical experience in treating subacute facial nerve injuries. These cases highlight nerve graft as an appropriate treatment for subacute facial nerve injuries.
A 66-year-old woman visited the clinic and presented with facial asymmetry secondary to nerve paralysis. Following a midand lower-facelift surgery at a private clinic 10 days earlier, the patient noticed that her face had deviated to the left side. Upon examination, a left-sided deviation was observed when the patient was at rest. Moreover, she exhibited asymmetric facial expressions when smiling and pursing her lips, whereas other facial movements, such as brow elevation and eye closure remained unaffected (Fig. 1A). Subsequent nerve conduction study and electromyography detected no motor unit action potential in the right orbicularis oris or levator labii muscles. However, minor contractions were observed in the orbicularis oculi and mentalis muscles. These results suggested a complete transection, which corresponds to neurotmesis, Sunderland grade V of the buccal branch of the facial nerve, and an incomplete injury to the zygomatic and marginal mandibular branches (Tables 1, 2). Consequently, surgical intervention was recommended and performed 20 days after the trauma (Table 2). As expected, nerve degeneration and scarring was encountered due to the delay. During exploration, the proximal stumps of two severed nerves were identified. The corresponding distal nerve ends were located by tracing the nerve backwards from the muscle using a nerve stimulator. After measuring the defect length from the main trunk of the facial nerve to the zygomaticus and buccinator muscles, a 13 cm sural nerve graft was obtained from her right calf. The harvested nerve was split longitudinally to be placed into the defect areas and sutured to restore the continuity (Fig. 1B and C). The patient was discharged 7 days after the procedure. Minor improvements in the patient’s facial expressions were observed in subsequent examinations. Physical therapy was started 1 month after the procedure and gradual improvements in facial symmetry and movements were observed over an 8-month follow-up period (Fig. 1D and E).
A 63-year-old woman visited the emergency department with a 2.5 cm laceration and crush wound on her right temple. Because of excessive swelling in the right periorbital area, it was hard to properly inspect the patient’s facial movements during emergency care. The following day, the patient returned to the clinic with impaired facial movement on the right side of her forehead, whereas other facial expressions remained unaffected (Fig. 2A). The nerve conduction study results revealed amplitudes of 1.2 and 0.6 mA on her left and right orbicularis oculi, respectively, suggesting partial injury to the temporal branch of the facial nerve. Hence, surgical intervention was recommended and conducted 8 days after the trauma (Table 2), during which crushed soft tissues, including muscles and nerve branches, were observed under a microscope. Upon its location, the crushed nerve, which lacked continuity, was classified as grade V based on the Sunderland classification system (Tables 1, 2). Subsequently, a 3.8 cm-long sural nerve was harvested from the patient’s right lower leg, followed by nerve grafting to bridge the gap in the crushed area (Fig. 2B). No complications occurred and the patient was discharged after 2 days. The symmetry of the patient’s facial movements, including facial expressions, forehead wrinkling, and eyebrow elevation, gradually improved over a 3-month follow-up period to almost normal levels (Fig. 2C).
Facial nerve paralysis, which impairs facial muscles, compromises the expression of emotions and facial communication, thereby significantly affecting the quality of life. Moreover, it sometimes causes oral incompetence of speech and swallowing, depending on the affected nerve. Because trauma is the second most common cause of facial paralysis, plastic surgeons frequently encounter such cases in emergency departments and clinics [1,2]. Following facial nerve injury, intervention timing is critical because nerve degeneration starts immediately after nerve transection and progresses to the cicatricial phase. Hence, such injuries involve challenging treatment procedures and are associated with poor prognosis [5].
In some cases, deciding between observation and surgical intervention might be challenging, and the decision should be based on the degree and type of injury [1,6]. Seddon (1943) and Sunderland (1951) developed several peripheral nerve injury classification systems and surgical interventions are typically recommended for Sunderland grade IV injuries (Table 1) [7]. Based on this classification system, neuropraxia, a focal segmental myelin injury, leads to the complete recovery of nerve function. However, axonotmesis and neurotmesis, which include axonal injury, eventually undergo Wallerian degeneration. Furthermore, there are differences between axonotmesis and neurotmesis. For instance, axonotmesis, which is not a complete transection, involves a partial or preserved conduction block with some degree of amplitude, even when nerve conduction study or electromyography reveals abnormal findings. In contrast, neurotmesis, which involves complete transection, exhibits a complete conduction block without amplitude (Table 1). These classifications guide the development of treatment strategies based on injury severity (Table 1).
Suspected cases of iatrogenic facial paralysis because of nerve injury require urgent intervention and immediate direct repair is recommended. However, depending on the injury, nerve grafting can be considered [1]. Fliss et al. [2] categorized nerve injuries as acute (up to 72 hours after injury), subacute (72 hours to 12–18 months after injury), and long-standing (more than 12–18 months after injury). Some studies have recommended nerve repair for up to 12 months after the injury [2,3]. A study by Hu et al. [8], which compared the passing rates of myelinated fibers in two groups of guinea pigs that were subdivided into several groups depending on the timing of facial nerve repair by direct suturing, found no statistical differences between the groups that underwent immediate nerve suture and those that received delayed suture by up to 14 days. However, the passing rates of myelinated fibers were lowest in the groups that received 60- and 90-day repair delays. Yuguchi et al. [9] observed decreased growth inhibitory factor mRNA levels between the third day of the injury and 5 weeks after the injury. However, a different study reported that the expression of glial cell line-derived neurotrophic factor peaked one week after injury and fell to the basal level over the following 6 months [10]. Based on these observations, Hu and colleagues recommended that the timing of facial nerve repair should not exceed 60 days [9]. Moreover, Yawn et al. [11] reported that the prognosis of long-term facial function differs significantly between the early (within 14 days) and delayed (after 14 days) repair groups. The early repair group experienced better outcomes, with a House–Brackmann grade of at least 3 at the final follow-up. Taken together, these studies indicate that despite delayed intervention, nerve repair or reconnection through grafting might be effective if performed within the first 6 months of the injury [2,12].
In the cases reported here, sural nerve grafting surgery was performed in the subacute phase of injury and direct repairs were not feasible because of the gaps between the proximal and distal stumps. Sural nerve grafting, which has several advantages, including sufficient length and low donor site morbidity, has been previously used to bypass areas of discontinuity [13]. A similar prognosis can be achieved using different repair techniques, such as end-to-end direct suturing, side-to-end nerve grafting, and interposition bypass grafting [4,12,14].
As presented above, some cases of nerve damage require nerve grafting. Even though grafting is more time consuming than direct repair and creates donor site morbidity, it is inevitable when nerve tension occurs [15]. This report includes the procedure of axial splitting of the harvested nerve. Nerve splitting is performed for diverse occasions, such as to minimize the donor site morbidity or to restore a fine branching structure of the facial nerve [16-18].
As discussed earlier, early direct repair is crucial for effective traumatic nerve injury restoration. Here, we present two cases of facial nerve paralysis that achieved favorable outcomes after surgical intervention using nerve grafts. In these cases, surgery was slightly delayed and direct nerve reconnection as not feasible. Nevertheless, they highlight nerve grafting in the subacute phase of the injury as an alternative treatment for facial nerve injury, which warrants further investigation.
Notes
Ethical approval
The study was approved by the Institutional Review Board of Kangdong Sacred Heart Hospital (IRB No. KANGDONG 2022-08-021).
Fig. 1.
A 66-year-old woman with facial paralysis after undergoing facelift surgery. (A) A photograph 19 days after iatrogenic injury showing aggravated facial expression asymmetry. (B, C) Intraoperative photographs of the nerve grafts. (B) Nerve coaptation between the harvested sural nerve and the proximal facial nerve (black arrow). (C) Coaptation with the distal nerve branches (blue arrows). (D, E) Photographs at 8 months after nerve grafting. (D) Symmetrical face movements during diverse facial expressions. (E) Significantly improved lip pursing.
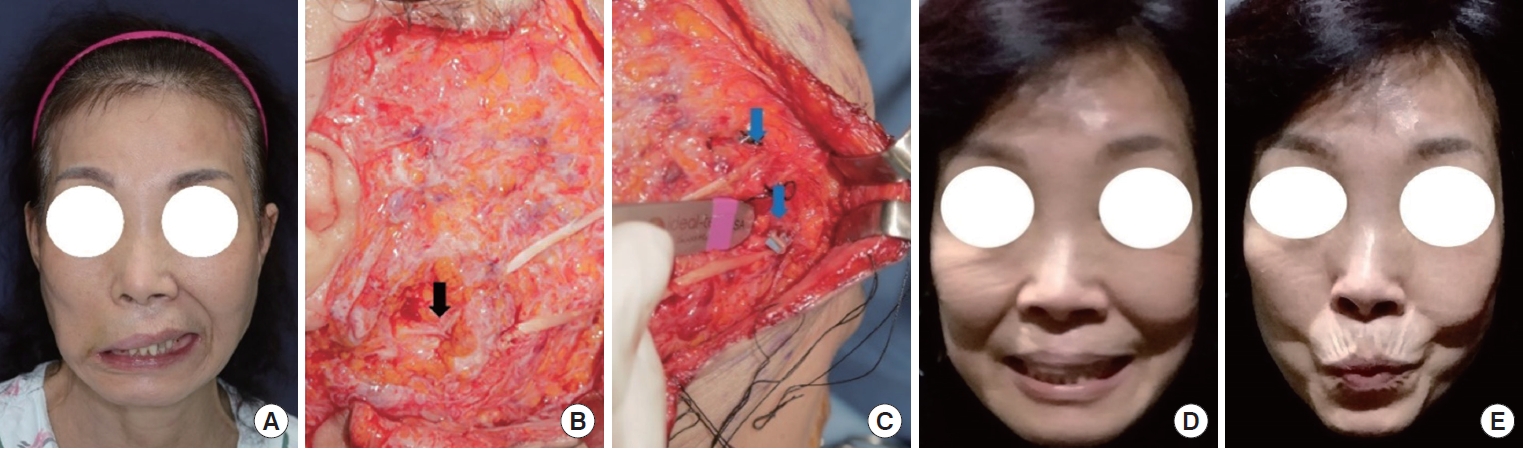
Fig. 2.
A 63-year-old woman with asymmetric brow movements after a crush injury to her right temple. (A) Impaired right brow elevation. (B) An intraoperative photograph. A nerve graft crossing over the injury from the proximal nerve stump to the distal nerve fiber entering the frontalis muscle. (C) Postoperative 3 months. Symmetrical brow elevation with apparent forehead wrinkling.
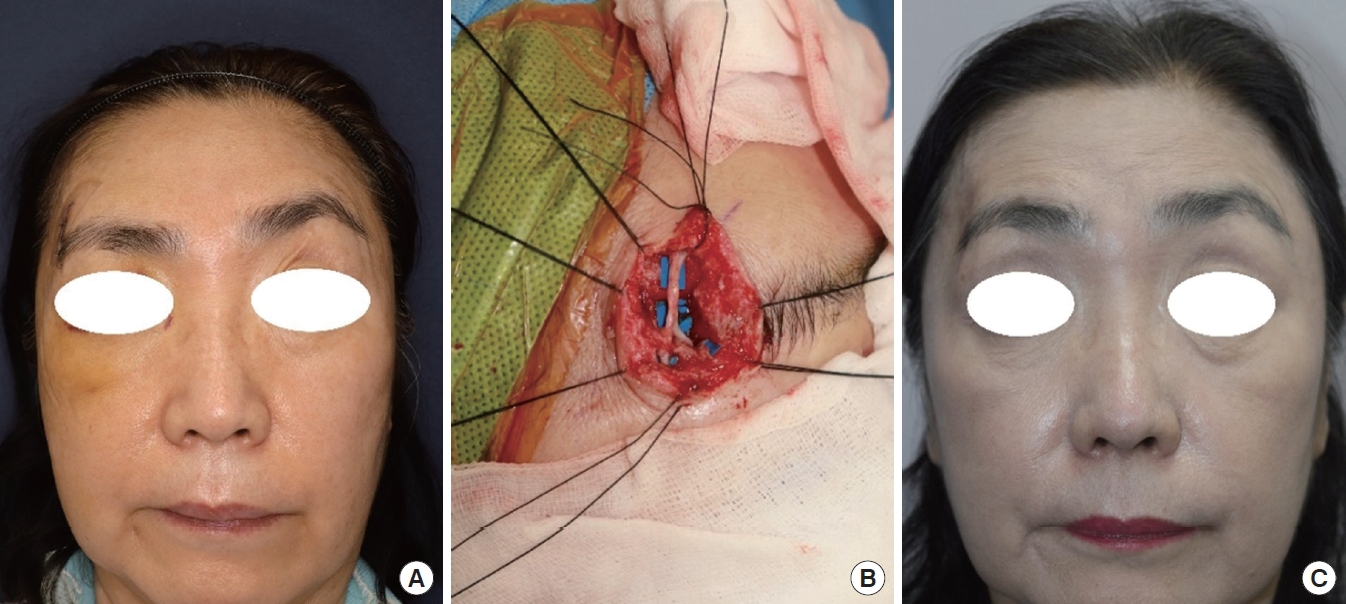
Table 1.
Seddon and Sunderland classification of peripheral nerve injury
Table 2.
Patient demographics
| Variable | Case 1 | Case 2 |
|---|---|---|
| Age (yr) | 66 | 63 |
| Sex | Female | Female |
| Injury type | Iatrogenic | Traumatic |
| Seddon and Sunderland classification | ||
| Seddon | Neurotmesis | Neurotmesis |
| Sunderland | V | V |
| Affected facial nerve branch | Zygomatic buccal | Frontal |
| Underlying disease | Hypertension | None |
| Smoking | None | None |
| Use of steroids | Dexamethasone 5 mg for 7 day | Dexamethasone 5 mg for 3 day |
| Surgery timing (day)a) | 20 | 8 |
| Surgical procedure | Sural nerve graft | Sural nerve graft |
| Hospital stay (day) | 12 | 4 |
REFERENCES
1. Walker NR, Mistry RK, Mazzoni T. Facial nerve palsy. In: StatPearls [Internet]. StatPearls Publishing; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK549815/.
2. Fliss E, Yanko R, Zaretski A, Tulchinsky R, Arad E, Kedar DJ, et al. Facial nerve repair following acute nerve injury. Arch Plast Surg 2022;49:501-9.



3. Brown S, Isaacson B, Kutz W, Barnett S, Rozen SM. Facial nerve trauma: clinical evaluation and management strategies. Plast Reconstr Surg 2019;143:1498-512.


4. Jandali D, Revenaugh PC. Facial reanimation: an update on nerve transfers in facial paralysis. Curr Opin Otolaryngol Head Neck Surg 2019;27:231-6.


5. Wang ML, Rivlin M, Graham JG, Beredjiklian PK. Peripheral nerve injury, scarring, and recovery. Connect Tissue Res 2019;60:3-9.


6. Odebode TO, Ologe FE. Facial nerve palsy after head injury: case incidence, causes, clinical profile and outcome. J Trauma 2006;61:388-91.


7. Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain 1951;74:491-516.


8. Hu J, Zhou L, Ma Z. Delayed repair of facial nerve trauma: an experimental study in guinea pigs. Acta Otolaryngol 2013;133:772-8.


9. Yuguchi T, Kohmura E, Yamada K, Sakaki T, Yamashita T, Otsuki H, et al. Changes in growth inhibitory factor mRNA expression compared with those in c-jun mRNA expression following facial nerve transection. Brain Res Mol Brain Res 1995;28:181-5.


10. Hoke A, Gordon T, Zochodne DW, Sulaiman OA. A decline in glial cell-line-derived neurotrophic factor expression is associated with impaired regeneration after long-term Schwann cell denervation. Exp Neurol 2002;173:77-85.


11. Yawn RJ, Wright HV, Francis DO, Stephan S, Bennett ML. Facial nerve repair after operative injury: impact of timing on hypoglossal-facial nerve graft outcomes. Am J Otolaryngol 2016;37:493-6.


12. Li L, Fan Z, Wang H, Han Y. Efficacy of surgical repair for the functional restoration of injured facial nerve. BMC Surg 2021;21:32.




13. Azizzadeh B, Irvine LE, Yoo DB, Larian B, Massry GG, Peng GL. Single-incision sural nerve harvest: technical considerations for cross-facial nerve grafting. Laryngoscope 2019;129:2464-6.



14. Condie D, Tolkachjov SN. Facial nerve injury and repair: a practical review for cutaneous surgery. Dermatol Surg 2019;45:340-57.


15. Sun S, Lu D, Zhong H, Li C, Yang N, Huang B, et al. Donors for nerve transplantation in craniofacial soft tissue injuries. Front Bioeng Biotechnol 2022;10:978980.










