The effect of biphasic calcium phosphate and demineralized bone matrix on tooth eruption in mongrel dogs
Article information
Abstract
Background
Bone grafts can provide an optimal environment for permanent tooth to erupt and enhance the stability of the alveolar maxilla. Although autologous bone is an optimal source for osteogenesis, its inevitable donor site morbidity has led to active research on bone substitutes. This study was designed to evaluate the safety and feasibility of using biphasic calcium phosphate (BCP; Osteon) as a bone substitute in dogs.
Methods
Bilateral third and fourth premolars of four 15-week-old mongrel dogs were used. All teeth were extracted except the third premolar of the right mandible, which was used as a control. After extraction of the premolars, each dog was administered BCP (Osteon), demineralized bone matrix (DBM; DBX), and no graft in the hollow sockets of the right fourth premolar, left fourth premolar, and left third premolar, respectively. Radiographs were taken at 2-week intervals to check for tooth eruption. After 8 weeks, each dog was sacrificed, and tooth and bone biopsies were performed to check for the presence of tooth and bone substitute particle remnants.
Results
Four weeks after the operation, permanent tooth eruptions had started at all the extraction sites in each dog. Eight weeks after the operation, all teeth had normally erupted, and histological examination revealed BCP particles at the right fourth premolar.
Conclusion
In all four dogs, no delay in the eruption of the teeth or shape disfigurement of permanent teeth was observed on gross inspection and radiologic evaluation. On histological examination, most of the BCP and DBM were replaced by new bone. Bone substitutes can be used as graft materials in patients with alveolar clefts.
INTRODUCTION
Patients with cleft lip/palate may present with abnormalities of the bony structure [1,2], more commonly with a cleft in the alveolar bone. An autologous cancellous bone graft placed in the alveolar cleft can provide a suitable route for tooth eruption, achieve stability of the alveolar ridge, and augment the periodontal space and nostril sill. An appropriate eruption cannot be expected without adequate bone tissue support. Autologous cancellous bone graft from the anterior superior iliac spine is the gold standard for bone conduction and induction, with minimal immune rejection or bone resorption. However, complications, such as hematoma, pain, scar, limping, or numbness of the anterolateral thigh due to lateral femoral cutaneous nerve injury, have been reported as donor site morbidities [3]. Therefore, research has been conducted to find optimal alternatives to autologous bone.
Synthetic alloplastic materials are under active investigation and have been successfully applied in various clinical situations. They are biologically inert and provide a favorable environment for bone regeneration [4] while minimizing the fear of infectious disease transmission, as in the case of allografts or xenografts [5]. Biphasic calcium phosphate (BCP), which is an 8:2 mixture of hydroxyapatite and β-tricalcium phosphate developed by Nery et al. [6] is now being widely applied in the fields of orthopedics and dental medicine as a favorable substitute for autografts, allografts, and xenografts. Some studies have shown demineralized bone matrix (DBM), another good source of graft material, to have better outcomes than BCP in bone defect models [7]. The bone morphogenic protein (BMP) contained in DBM is known to play a key role in osteoinduction.
However, the effectiveness of bone substitutes in tooth eruption has not yet been elucidated. Many previous studies have reported the negative effects of bone substitutes on tooth eruption [8-10]. Incomplete eruption or tooth deformation was observed when bone substitutes were implanted instead of autologous bone. Hydroxyapatite particles are thought to be a major obstacle in tooth eruption. In this study, commercially available BCP containing less hydroxyapatite (Osteon) was used to reduce its negative effects. BCP (Osteon) is composed of 70% hydroxyapatite and 30% β-tricalcium phosphate.
To confirm the hypothesis that bone substitutes/BCP (Osteon) do not interfere with tooth eruption, we investigated the effects of BCP and DBM on tooth eruption in mongrel dogs. Radiologic and histologic evaluations were performed to elucidate the effects of BCP and DBM on permanent tooth eruption. We assessed the possibility of clinical application of bone substitutes in the alveolar cleft as a suitable alternative to autologous bone.
METHODS
Materials
The BCP used in this study was Osteon (Genoss, Suwon, Korea), which is composed of 70% hydroxyapatite and 30% β-calcium phosphate [11]. BCP (Osteon) has two product lines of different particle sizes: 0.5–1.0 mm and 1.0–2.0 mm; the smaller size was adopted for the present study. For DBM, putty form DBX (Synthes, West Chester, PA, USA) was used, which is made of 32% DBM from cadavers cross-linked with sodium hyaluronate.
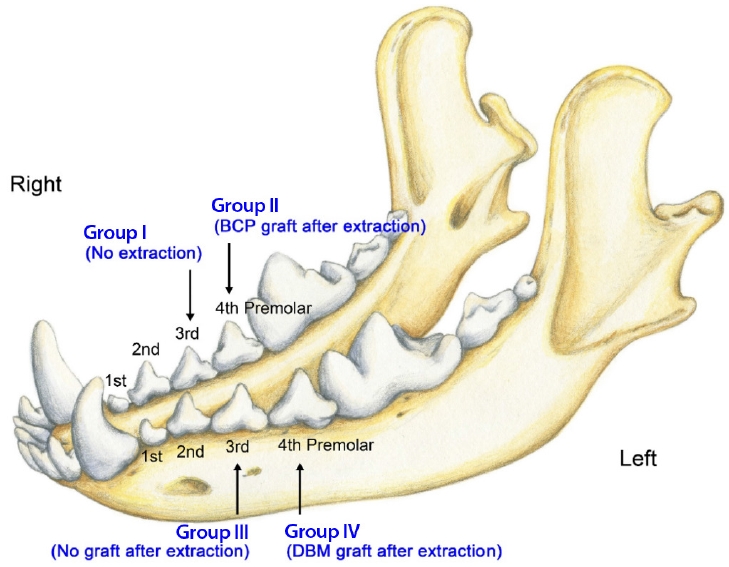
Four 13-week-old mongrel dogs were prepared for the explorative animal study because there are no previous reports or pilot studies of the same experimental model. In mongrel dogs, deciduous teeth erupt at 4–8 weeks of age, permanent incisors at 20–24 weeks, permanent premolars at 16–24 weeks, and permanent molars at 16–28 weeks [12]. There is usually little difference in tooth eruption time between animals, and sufficient time for graft uptake is secured between the eruption time of deciduous and permanent teeth. They were acclimated for 2 weeks in the laboratory animal breeding facilities under controlled temperature, humidity, atmosphere, and light exposure. The experiment was performed with animals aged 15 weeks. The mandibular third and fourth premolars were assigned to four groups. The third premolar on the right side was used as the negative control group (group I), the fourth premolar on the right side was used for the BCP graft group (group II), the third premolar on the left side was used as the positive control group (group III), and the fourth premolar on the left side was used for the DBM graft group (group IV) (Fig. 1).
Methods
Food or water was restricted overnight upon induction of general anesthesia. Preoperative radiography was performed to check the eruptive status of the tooth after sleep induction with 80 mg/kg ketamine and 10 mg/kg xylazine. Anesthesia was continued using isoflurane gas under orotracheal intubation, and the teeth were extracted after an injection of 20 mg/kg cefazoline into the subcutaneous layer. The fourth premolar on the right side, and the third and fourth premolars on the left side were extracted using dental extraction forceps. The remnant tooth root was removed using a small size drill if en bloc extraction failed. Care was taken to not expose or injure the permanent teeth.
The right third premolar was not extracted in group I, which formed the negative control. In group II, BCP was compactly grafted into the defect space after extraction, thereby repairing the graft site. Mucoperiosteal flaps were elevated from both the labial and lingual sides and sutured together using 4-0 Vicryl. No graft following extraction was performed in group III, which comprised the positive control. DBM was grafted similarly as in group II, at the left fourth premolar tooth in group IV. Postoperative radiographs were obtained immediately to check for the status of the grafted bone substitutes and any injury to the permanent teeth. Cefazoline (20 mg/kg) was subcutaneously injected twice a day for 5 days to prevent surgical site infection, and meloxicam (0.4 mL/kg) was injected into the subcutaneous layer once a day for 5 days to alleviate postoperative pain. We checked the operation sites twice a day for 7 days when sterilizing the wound with 0.1% chlorhexidine solution. Oral intake was permitted from the day after the operation, and solid food soaked in water was supplied for 7 days.
Gross and radiologic evaluation
The eruption of permanent teeth was observed grossly and with radiological evaluation. Radiographs were obtained immediately postoperatively and at 2, 4, and 6 weeks after the operation under sedative anesthesia. Various factors were investigated, including the degree of tooth eruption, general appearance of the erupted tooth, elongation of the tooth root, and the status of the grafted BCP and DBM.
Degree of eruption of permanent teeth
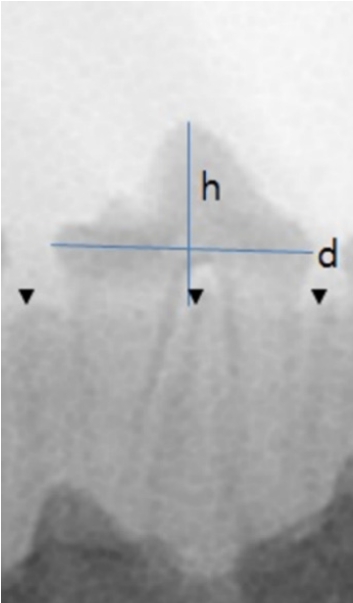
The degree of eruption was measured using radiography. The distance between the upper border of the alveolar ridge and the top of the crown (h) was determined as an index of eruption. The value of “h” was divided by the maximal diameter of the crown (d) to correct the change in absolute value according to the distance and angle from the X-ray source (h/d) (Fig. 2). The value was considered negative when the eruption had not yet occurred, and the tooth was embedded in the alveolar bone.
Histological evaluation
The animals were sacrificed at 8 weeks after the operation, and the third and fourth premolars, including the mandible, were harvested. The specimens were fixed in a 10% formalin solution overnight without demineralization. After dehydration with 80% alcohol, they were placed in a methyl methacrylatebased resin. The methyl methacrylate-based resin dissolved in 1.4 g benzoyl peroxide was applied and changed twice within a time interval of 2 days. Benzoyl peroxide (3.5 g) was prepared using the same method. After embedding, the biopsy slide was prepared from the resin block, which included the mid-plane of the tooth. Hematoxylin-eosin staining was performed, and the slides were reviewed in terms of the cutting edge of the tooth, resorption of bone substitutes, and new bone regeneration.
Statistical analysis
The degree of eruption was compared postoperatively at 2, 4, and 6 weeks after the operation using the Friedman test. Statistical significance was set at p<0.05. The Wilcoxon signed-rank sum test was used for post-hoc analysis when the Friedman test revealed statistical differences among the four groups.
RESULTS
Gross and radiological evaluation
Immediate postoperative radiography revealed radiopaque densities in the BCP graft group (group II) and radiolucent densities in the DBM graft group (group IV). Permanent teeth were found to be impacted in the alveolar bone in all groups (Fig. 3). Two weeks after the operation, when the dogs were 17 weeks old, the deciduous tooth of group I had still did not shed. Some of the permanent teeth in groups II, III, and IV started to erupt, perforating the overlying mucoperiosteum, and their location and appearance were normal. BCP was observed over the permanent tooth, although the radiopaque densities were attenuated (Fig. 4).
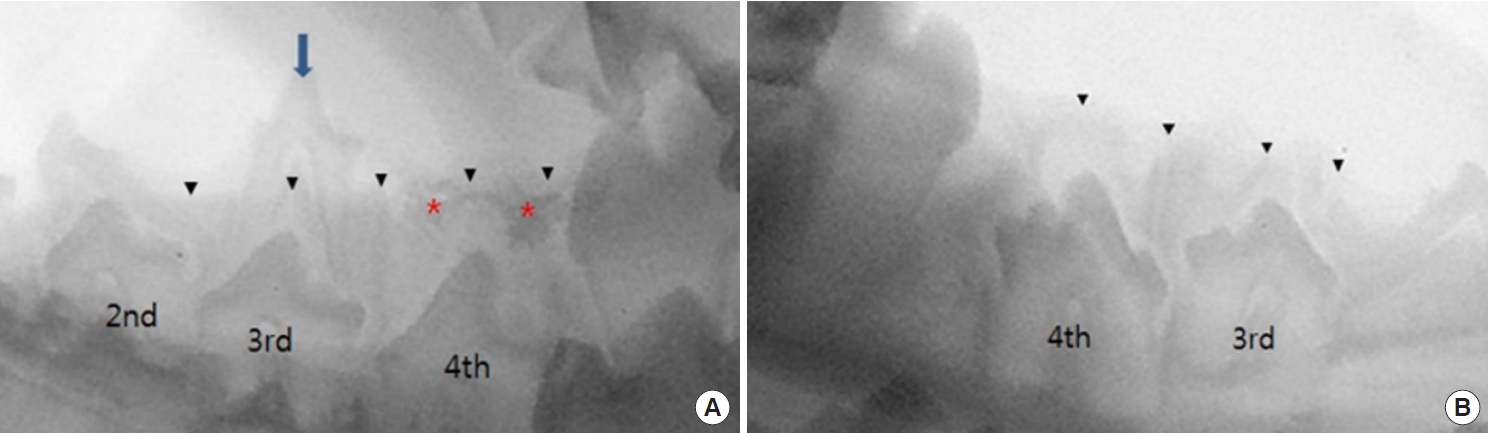
Immediate postoperative radiographic images. (A) The deciduous third premolar of right mandible is maintained and the hollow socket at fourth premolar site of right mandible is packed with biphasic calcium phosphate (BCP), which is radiopaque. (B) The hollow socket of the third premolar of left mandible is not grafted and the hollow socket of the fourth premolar is packed with demineralized bone matrix which is radiolucent. Blue arrow indicates the right third premolar deciduous tooth. Black arrowheads indicate the upper margin of alveolar ridge, while red asterisks indicate radiopaque BCP.
Radiographic images at postoperative 2 weeks. (A) The deciduous third premolar of right mandible is not exfoliated. Biphasic calcium phosphate (BCP) at fourth premolar site is seen above a permanent tooth. (B) The permanent third and fourth premolars of left mandible have erupted beyond gingiva. Blue arrow indicates the right third premolar deciduous tooth. Black arrowheads indicate the upper margin of alveolar ridge, while red asterisks indicate radiopaque BCP.
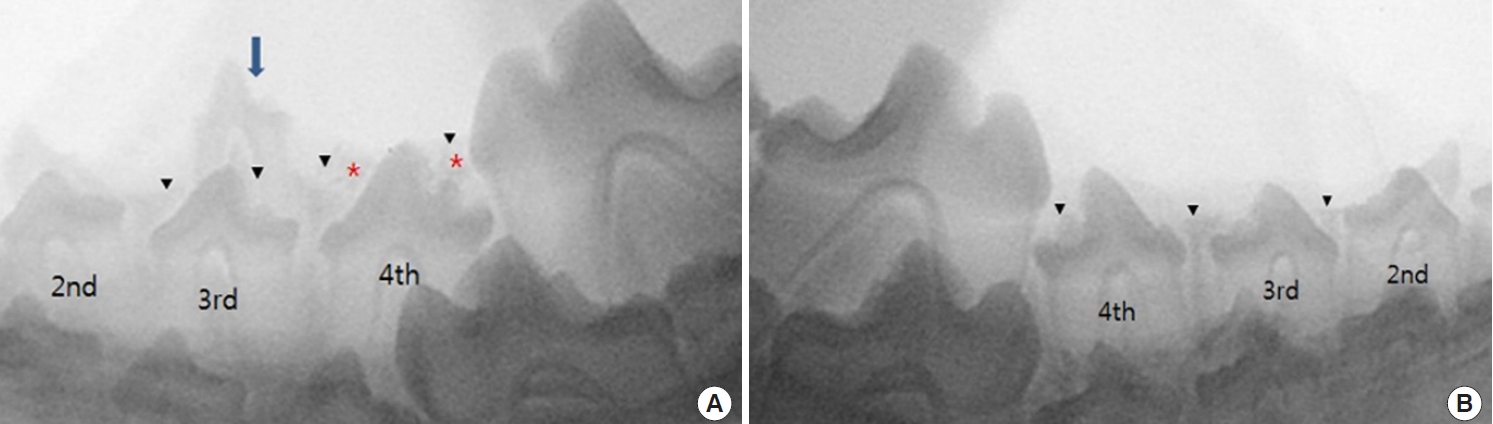
At postoperative week 4, when the dogs were 19 weeks old, the deciduous teeth of group I fell out, and the permanent teeth in all groups started to erupt. BCP was resorbed and partially seen in the periphery of the permanent tooth. The eruptions in groups III and IV showed no difference from those in the other groups (Fig. 5).

Radiographic images at postoperative 4 weeks. (A) The deciduous third premolar of right mandible is exfoliated. Biphasic calcium phosphate (BCP) at fourth premolar site seen around a permanent tooth crown. Two permanent teeth pierce gingiva. (B) The permanent third and fourth premolars of left mandible have erupted farther compared with the images taken 2 weeks before. Black arrowheads indicate the upper margin of alveolar ridge, while the red asterisk means radiopaque BCP.
At postoperative 6 weeks, when the dogs were 21 weeks old, eruption and elongation of the tooth root in all permanent teeth were observed. BCP was almost resorbed and scarcely visible on the mucosa between the teeth (Fig. 6).
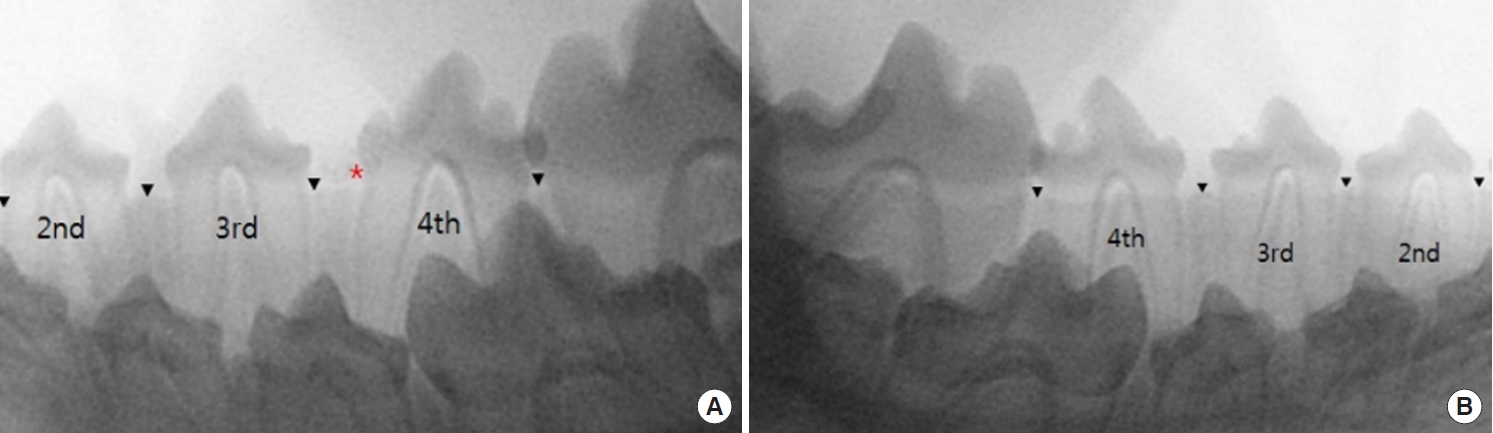
Radiographic images at postoperative 6 weeks. (A) The permanent third and fourth premolars of right mandible have erupted. Biphasic calcium phosphate (BCP) at fourth premolar site is seen around permanent tooth slightly. (B) The permanent third and fourth premolars of left mandible have erupted further. Roots are elongated compared with the images taken 2 weeks before. Black arrowheads indicate the upper margin of alveolar ridge, while red asterisk means radiopaque BCP.
At postoperative week 8, when the dogs were 23 weeks old, the eruptions of all permanent teeth were completed without any delay or deformities.
Eruption degree of permanent teeth
The BCP and DBM graft groups showed a similar pattern of eruption as the control groups. Permanent teeth started to erupt through the gingiva at 2 weeks, and the maximal amount of eruption was observed during the first 2 weeks. The amount of eruption started to decrease after 2 weeks and then noticeably dropped after 4 weeks (Fig. 7). There was no statistical difference in the degree of eruption postoperatively among the four groups, either immediately (p=0.589) or 2 weeks after (p=0.086). At 4 weeks after surgery, a statistically significant difference was observed (p=0.044). However, no significant difference was found between any two groups when post-hoc analysis was performed using the Wilcoxon signed-rank sum test. A small number of specimens was thought to be the cause of the weak power of the test. At 6 weeks after surgery, there were no significant differences in the degree of eruption among the four groups (p=0.127).
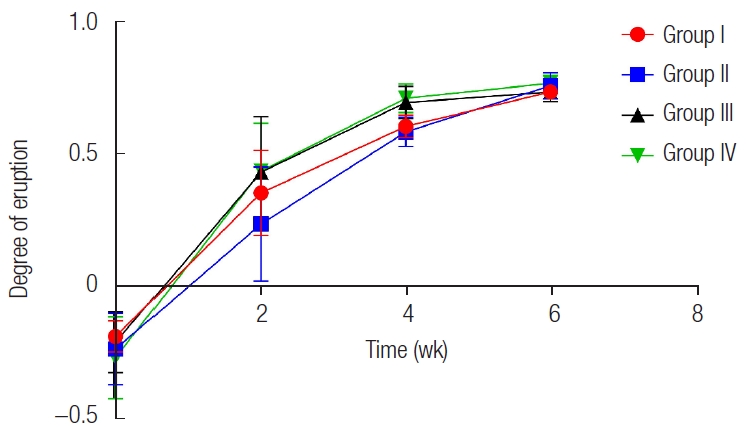
Eruption degree of permanent teeth. Scores on the vertical axis indicate the degree of eruption (h/d). The slopes of the graph indicate the velocity of tooth eruption. There was no significant difference in the eruption degree between groups except at postoperative 4 weeks. h, height from the alveolar ridge to the crown tip; d, maximal dimension of the crown.
Histological evaluation
The biopsy slide was made parallel to the mandibular cortex for easy evaluation of the tooth cross-section. All permanent teeth showed normal structure, and the alveolar bone was well attached to the tooth without defects. The DBM graft in group IV was resorbed and replaced by the alveolar bone. A small portion of the BCP in group II was observed in the periphery, but this was not apparent (Fig. 8). However, particles of BCP that were not absorbed and could be confirmed with the naked eye, were observed in the mucosa.

Histological evaluation. (A) Non-graft site. Normal tooth and alveolar bone (H&E, ×40). (B) Demineralized bone matrix (DBM) graft site. No difference from non-graft site and DBM particles are seen (H&E, ×40). (C) Biphasic calcium phosphate (BCP) graft site. Large particles of BCP (yellow arrow) were observed near the tooth (H&E, ×40). (D) Another BCP graft site. A small particle of BCP (yellow arrow) is seen in the alveolar bone (H&E, ×80).
DISCUSSION
The results of the present study revealed that there was no delay in eruption or deformity of teeth when BCP or DBM was grafted. A temporary delay in eruption was observed at 4 weeks after the surgery in the BCP graft group. It is thought that hydroxyapatite in BCP acts as a barrier for tooth eruption, but normal eruption was obtained at 6 weeks in all groups. The graph of the eruption shows a similar pattern among the four groups. The degree of tooth eruption was remarkable during the first 2 weeks and then decreased afterward. This is an acceptable result considering the fact that the premolar permanent teeth of mongrel dogs erupt during 16–24 weeks [12].
The autologous bone graft group was not included in this study because of the relatively small defect size after tooth removal, donor site morbidity, and stress on the animals. Group III, in which no graft was placed after tooth removal, could be considered as positive control. Deciduous tooth extraction did not affect the eruption of permanent teeth in groups II and IV as well as in group III.
It is generally thought that hydroxyapatite in BCP has a negative effect on permanent tooth eruption. Holtgrave [9] extracted deciduous teeth in 5-day-old mice and implanted hydroxyapatite of different particle sizes, and reported loss of tooth eruption. Feinberg and Vitt [8] developed a similar defect model in cats aged 3–4 months, and implanted hydroxyapatite and β-tricalcium phosphate. Both studies found an appropriate eruption in the β-tricalcium phosphate-implanted group, but delayed eruption and deformation of the tooth in the hydroxyapatite- implanted group. Therefore, they concluded that the alveolar bone above the permanent tooth should be resorbed for a successful eruption, and that non-biodegradable hydroxyapatite can interfere with tooth eruption, leading to a delay in eruption or deformation of the tooth. A relatively small portion of hydroxyapatite (70%) in BCP (Osteon) used in the present study did not interfere with permanent tooth eruption. Biodegradation of β-tricalcium phosphate in BCP (Osteon) released hydroxyapatite, and hydroxyapatite moved to the periphery making way for the permanent tooth. Sugimoto et al. [13] described the mechanism of permanent tooth eruption using bone substitutes. First, bone resorption occurs around the follicle of a permanent tooth and the more distal region. Second, the complex of new bone and bone substitutes is dissolved in the process of new bone resorption. Third, separated substitutes from new bone are discharged into the peripheral area by the pressure of eruption. The results of the present study concur with Sugimoto’s opinion. The normal eruption of permanent teeth and bone substitutes replaced by new bone can be explained by the first and second mechanisms. The histological result that reveals a mass of BCP in the periphery of the graft is evidence of the third mechanism.
DBM contains non-collagenous proteins, such as BMP, and produces bone morphogenic factors. DBM causes osteoinduction and partial osteoconduction, but it is used as an adjuvant treatment for promoting osteogenesis because it does not contain osteogenic cells. MacIsaac et al. [10] performed a retrospective study to compare tooth eruption between the two groups of autologous cancellous bone grafts and the other consisting of a mixture of autologous cancellous bone, DBM, and allografts. They found an increased incidence of incomplete or non-eruption in the latter group. In the present study, a normal eruption was observed in the DBM graft group.
In previous studies, efforts have been made to create an alveolar cleft simulation in animal models. Mayer et al. [14] created a bony defect in foxhound dogs by extracting maxillary incisors and drilling them with a burr. Kamal et al. [15] extracted the central incisor in New Zealand white rabbits to form a pocketlike cavity. Based on these studies, we used mongrel dogs (15-week-old of age) to surgically establish the alveolar cleft model by extracting the teeth.
However, compared to the amount of graft material in patients with alveolar cleft, the amount placed in the remaining defect area after tooth extraction is relatively small. In terms of the structure of the alveolar bone, the number of teeth and timing of tooth eruption are similar in animals and humans. Although the results obtained from the dog eruption model cannot be directly applied to the alveolar cleft in humans, the fact that the bone substitute can be replaced with new bone without suppressing the eruption of permanent teeth may have clinical significance and can be considered as an alternative to autologous bone grafts for patients with alveolar cleft. In clinical practice, patients with alveolar clefts do not have proper eruption of their permanent teeth unless bone grafting is performed. Further studies should be performed to create a realistic animal model of alveolar cleft and to investigate the effect of bone substitutes on the alveolar gap. Through this, it will be possible to enhance the clinical significance of bone substitutes as an alternative to autogenous bone grafts.
There are some limitations to the present study. First, the sample size was relatively small, and the groups were not independent. Statistical analysis was chosen considering this point, but a small sample size can result in a statistical error. Second, we were not able to create an actual alveolar cleft model; therefore, a tooth eruption model was adopted instead. It is unreasonable to apply the results of this study to the alveolar cleft directly, as the route for tooth eruption is longer and the amount to be grafted is larger in the alveolar cleft. The results of this study show the possibility of using a BCP (Osteon) graft as a substitute for autologous grafts. Further research should be conducted to evaluate the efficacy of BCP (Osteon) for normal tooth eruption in the alveolar cleft.
In conclusion, the normal eruption of permanent teeth in dogs was not affected by BCP or DMB grafting when they were grafted into the dead space following the extraction of deciduous teeth. These results suggest the possibility of its clinical use in the alveolar clefts. The effectiveness and safety of BCP (Osteon) must be investigated in future studies including human alveolar clefts.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was approved by the Institutional Animal Care and Use Committee of Seoul National University (IACUC No. 09- 0057).
Author contribution
Conceptualization: Ki Yong Hong, Sukwha Kim. Formal analysis: Si Woo Lee, Byung Jun Kim. Methodology: Ki Yong Hong, Tae Hyun Choi. Project administration: Ji-Young Kim. Visualization: Byung Jun Kim. Writing - original draft: Si Woo Lee, JiYoung Kim, Ki Yong Hong. Writing - review & editing: Si Woo Lee, Ji-Young Kim, Byung Jun Kim. Resources: Tae Hyun Choi. Supervision: Sukwha Kim. All authors read and approved the final manuscript.
Abbreviations
BCP
biphasic calcium phosphate
BMP
bone morphogenic protein
DBM
demineralized bone matrix