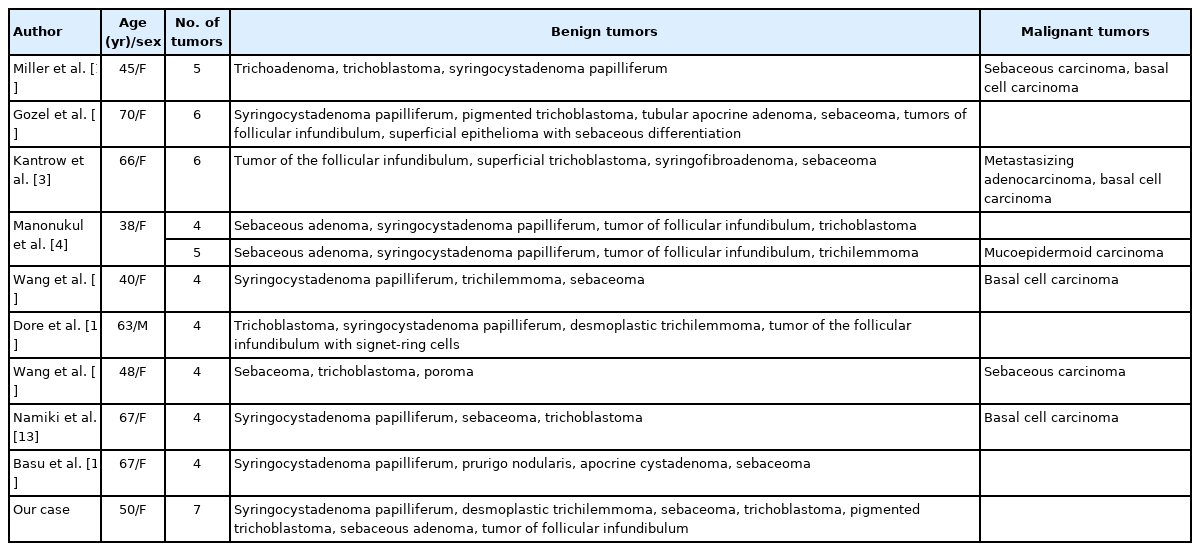
Development of seven secondary neoplasms in a nevus sebaceous: a case report and literature review
Article information
Abstract
Nevus sebaceous of Jadassohn is a congenital cutaneous hamartoma with epidermal, sebaceous, follicular, and apocrine structures that usually appears at birth or in early childhood. It has the potential to generate a variety of secondary neoplasms of different lineages, and the risk increases with patient age. Although multiple neoplasms may occasionally arise within the same lesion, the coexistence of more than five secondary tumors is extremely rare. Here we report a case of seven secondary tumors including syringocystadenoma papilliferum, desmoplastic trichilemmoma, sebaceoma, trichoblastoma, pigmented trichoblastoma, sebaceous adenoma, and tumor of follicular infundibulum arising within a nevus sebaceous. The complete diagnosis relies on the histopathological analysis of multipoint biopsies and delicate pathological sections.
INTRODUCTION
Nevus sebaceous of Jadassohn (NSJ) is a congenital cutaneous hamartoma that usually appears shortly after birth. Initially, it presents as well-demarcated, yellowish to brownish, smooth, and hairless plaques. During puberty, morphologic change occurs and the lesions assume a thickened and verrucous appearance. Its clinical importance lies mostly in the potential for neoplastic transformation with an estimated incidence of 10% to 30% [1]. Although multiple neoplasms may occasionally arise within the same lesion, only four reports in the English literature describe the simultaneous emergence of five or more neoplasms [1-4]. We report the first case of seven secondary benign tumors within a single NSJ.
CASE REPORT
A 50-year-old woman presented with an asymptomatic, skin-colored, irregular linear plaque on her scalp. The lesion had appeared at birth and became enlarged and bled easily in recent years. She had no history of local trauma or topical or surgical treatment. She was receiving long-term prednisolone and azathioprine treatment for systemic lupus erythematosus. There were no accompanying neurological symptoms and no personal or family history of cutaneous or internal malignancies.
Physical examination revealed a 7× 1.5 cm, linear, skin-colored, alopecic verrucous plaque on her left temporoparietal scalp (Fig. 1), which contained several grayish-yellow papules and three erythematous ulcerated nodules. Under the clinical diagnosis of NSJ with possible malignant change, the lesion was totally excised with a 3 mm free margin for histopathological evaluation.

A linear, skin-colored, alopecic verrucous plaque measuring 7×1.5 cm on the scalp. The plaque contained three erythematous ulcerated nodules (white arrows and arrowhead) and several grayish-yellow papules.
Microscopic examination of the main lesion revealed acanthosis, hyperkeratosis, and papillomatosis of the epidermis. Sebaceous gland hyperplasia, immature hair follicles, and ectopic apocrine glands were present in the dermis, which were consistent with a diagnosis of NSJ. Histopathologically, two connecting ulcerative nodules (Fig. 1, arrows) showed features of partially intertwined syringocystadenoma papilliferum (SCAP) and desmoplastic trichilemmoma (DT) (Figs. 2, 3). Features of sebaceoma were detected in an ulcerated nodule in the central part of the lesion (Figs. 1 arrowhead, 4). Trichoblastoma (TB), pigmented TB, sebaceous adenoma and tumor of follicular infundibulum (TFI), which were separated by connective tissue stroma, were identified in the area containing grayish-yellow papules under higher magnification (Figs. 5-8).

Syringocystadenoma papilliferum demonstrating papillary structures lined by double layers of cuboidal epithelium and stroma containing numerous plasma cells (H&E, ×20).

Desmoplastic trichilemmoma comprising a well-defined lobular tumor with dense desmoplastic stroma occupying the central portion of the lesion. There is focal clear cell differentiation (black arrow) surrounded by a basement membrane. The irregular epithelial strands without cellular atypia are admixed in a desmoplastic stroma (white arrow) (H&E, ×40).
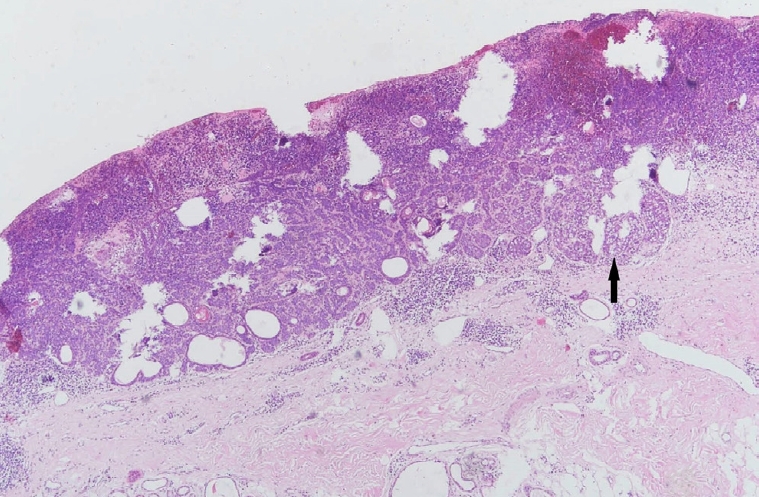
Sebaceoma demonstrating a basaloid dermal tumor with cyst formation, superficial ulceration, and hemorrhage. Ductal structures lined by an eosinophilic cuticle are visible. Note the random admixture of basaloid cells and mature sebocytes (black arrow) (H&E, ×40).
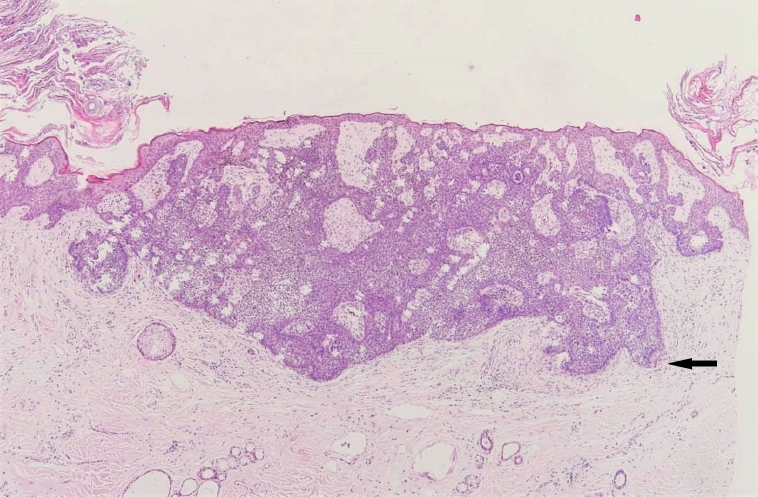
Trichoblastoma demonstrating basaloid proliferations with peripheral palisading emanating from the epidermis. Note the presence of papillary mesenchymal bodies (black arrow) and adjacent fibrotic stroma (H&E, ×40).
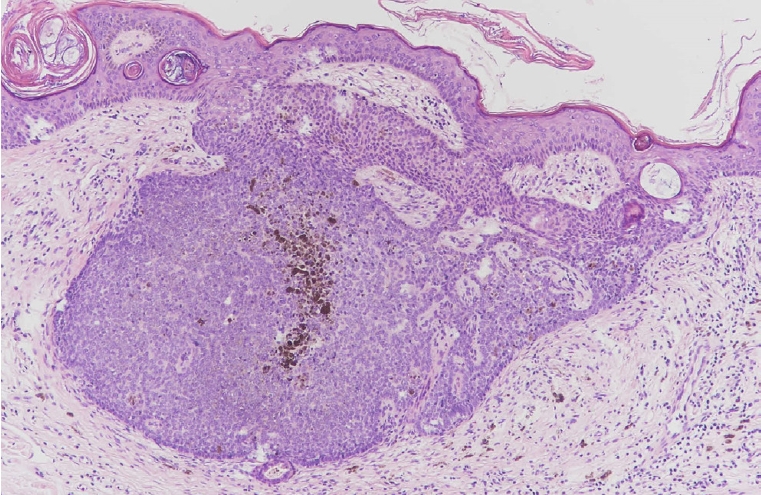
Pigmented trichoblastoma exhibiting melanin pigmentation within a typical trichoblastoma (H&E, ×200).
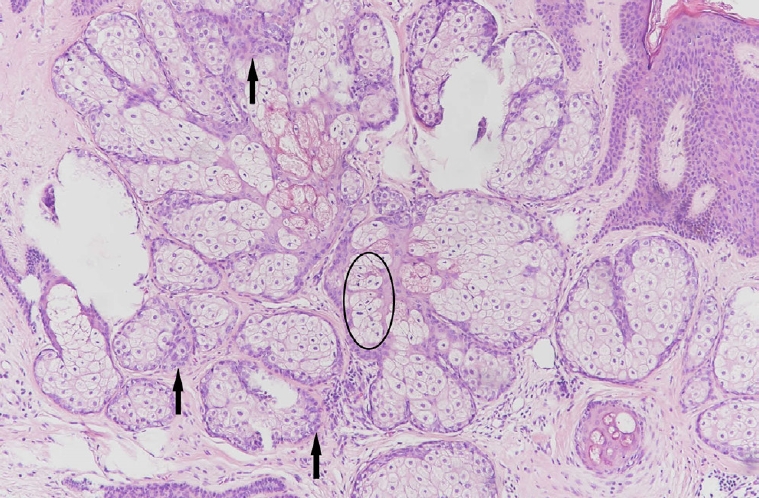
Sebaceous adenoma demonstrating a circumscribed tumor composed of irregular sebaceous lobules with surrounding basaloid cells more than two layers thick (black arrows). A few mitotic figures (black circle) are present in the central sebaceous lobule (H&E, ×100).
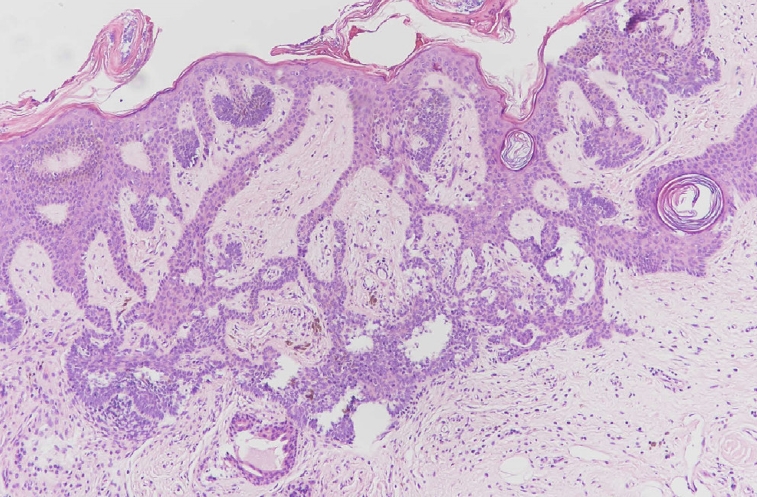
Tumor of follicular infundibulum comprising an epithelial tumor with a plate-like growth pattern. Note the anastomosing islands of basophilic cells with peripheral palisading and multifocal attachment to the overlying epithelium (H&E, ×200).
The simultaneous presence of two sebaceous neoplasms raised concern for Muir-Torre syndrome (MTS). The initial laboratory work-up for occult malignancy revealed a normal complete blood count, electrolytes, urine analysis, and renal and liver function. A further regular work-up was recommended to the patient, including colonoscopy, mammogram, pelvic exam, and chest X-ray. There was no recurrence of the scalp tumor 1 year after the surgery.
DISCUSSION
NSJs have the potential to generate a variety of secondary neoplasms of both sebaceous and non-sebaceous (e.g., follicular, apocrine, eccrine, muscle) lineages [5]. Histopathologically, it is also common to find proliferating basaloid cells or basaloid tumors within NSJs, which may be related to mutations in genes encoding PTCH and p53 [6,7]. As noted, NSJs rarely develop multiple secondary tumors simultaneously, and our patient appears to be the first such reported case containing seven secondary benign neoplasms with follicular, sebaceous, and sweat gland differentiation (Table 1).
According to previous studies, SCAP and TB are the most common benign tumors associated with NSJ [8]. Histopathologically, SCAP is composed of irregular papillary projections lined by double layers of glandular epithelium, with numerous plasma cells in the stroma. TB comprises benign basaloid epithelial cells with abundant fibrous stroma and focal clefts between collagen fibers. It should be differentiated from basal cell carcinoma (BCC), which is characterized by clefts between basaloid aggregations and surrounding mucinous stroma. In addition, TB typically contains papillary mesenchymal bodies, which are usually absent in BCC. Several immunohistochemistry markers (e.g., CD10, cytokeratin [CK] 15, CK19, CK20, Ber-EP4) can be used to assist in distinguishing benign follicular tumors from BCCs [9]. In our case, the lesion diagnosed as TB was focally positive for CK20 and negative for Ber-EP4.
DT is an uncommon histological subtype of trichilemmoma, and only 10 cases of DT arising within NSJ have been described [8,11,15,16]. Histopathologically, DT presents as a well-defined lobular tumor with typical features of trichilemmoma at the periphery and a central desmoplastic stroma. The presence of admixed irregular epithelial strands and nests in a dense sclerotic stroma may cause misdiagnosis as squamous cell carcinoma or morpheaform BCC. A correct diagnosis relies on the absence of cellular atypia and mitotic activity, and the presence of lobules of clear epithelial cells surrounded by an eosinophilic hyaline mantle. In difficult cases, CD34-positive epithelial strands may facilitate the differential diagnosis of DT over other desmoplastic tumors of the skin [17].
The NSJ in our patient also included areas resembling sebaceoma and sebaceous adenoma. Sebaceoma is composed of a random admixture of germinative basaloid cells and mature sebocytes lacking an organized lobular architecture [18]. Sebaceous adenoma comprises irregular sebaceous lobules with a rim of basaloid cells peripherally and mature sebocytes centrally [4]. Although neither neoplasm typically exhibits cytological atypia or a significantly increased mitotic rate, some benign sebaceous neoplasms in MTS are supposed to have a high potential for malignant transformation [19]. In our patient, there was no family history of internal malignancy, and the initial work-up for occult malignancy related to MTS yielded negative results. Further regular work-ups for cutaneous and internal malignancies may be warranted in this patient.
A TFI was also identified in our case, which is characterized by anastomosing islands of basophilic cells with multiple points of attachment to the overlying epithelium. The basaloid features and peripheral palisading in TFI may be similar to a superficial BCC or superficial portion of an infundibulocystic BCC. In contrast to BCC, the tumor cells in TFI are pale staining due to glycogen and lack hyperchromatic nuclei, peripheral clefting, and Ber-EP4 expression [2]. They may also include scattered CK20-positive Merkel cells, which are usually absent in BCC [20].
In conclusion, this report highlights the diverse neoplastic potential of NSJ toward adnexal tumors of different lineages and supports the hypothesis that NSJ develops from the pluripotent primary epithelial germ cells. The proliferation of these pluripotent germ cells into different lineages may explain the development of multiple secondary tumors. Clinically, for a longstanding NSJ with multiple tumor growth, multipoint biopsies and delicate pathological sections should be obtained to make a complete diagnosis and exclude the possibility of malignant transformation. It is important to select the appropriate biopsy site and size when evaluating the secondary neoplasms. Additional tissue sampling or pathological sections may be essential if the pathology findings do not entirely correlate with the clinical presentation. Although prophylactic excision may not be necessary for every adult patient with NSJ, close clinical surveillance and appropriate pathological examinations are warranted to monitor the development of neoplasms.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was approved by the Institutional Review Board of Kaohsiung Veterans General Hospital (IRB No. KSVGH20-CT12-21).
Patient consent
The patient provided written informed consent for the publication and the use of her images.
Author contribution
Data curation: Wei-Hsuan Tsai. Visualization: Yi-Wen Kuo, Jung-Chia Lin. Writing - original draft: Yi-Wen Kuo, Jung-Chia Lin. Writing - review & editing: Wei-Hsuan Tsai. Investigation: Yi-Wen Kuo, Jung-Chia Lin. Resources: Yi-Wen Kuo, WeiHsuan Tsai. Software: Jung-Chia Lin. Supervision: Wei-Hsuan Tsai.
Abbreviations
BCC
basal cell carcinoma
CK
cytokeratin
DT
desmoplastic trichilemmoma
MTS
Muir-Torre syndrome
NSJ
nevus sebaceous of Jadassohn
SCAP
syringocystadenoma papilliferum
TB
trichoblastoma
TFI
tumor of follicular infundibulum