Secondary bone grafting for alveolar clefts: surgical timing, graft materials, and evaluation methods
Article information
Abstract
Alveolar cleft belongs to the spectrum of cleft lip and/or palate, affecting 75% of cleft lip/palate patients. The goals of alveolar cleft treatment are stabilizing the maxillary arch, separating the nasal and oral cavities, and providing bony support for both erupting teeth and the nasal base via the piriform aperture. Secondary alveolar bone grafting is a well-established treatment option for alveolar cleft. Secondary alveolar bone grafting is performed during the period of mixed dentition using autologous bone from various donor sites. There are several issues relevant to maximizing the success of secondary alveolar bone grafting, including the surgical timing, graft material, and surgical technique. In this study, we reviewed issues related to surgical timing, graft materials, and evaluation methods in secondary alveolar bone grafting.
INTRODUCTION
Alveolar cleft belongs to the spectrum of cleft lip and/or palate, affecting 75% of such patients [1,2]. The gap of an alveolar cleft becomes wider from bottom to top, showing the widest bony gap at the piriform aperture, thereby resembling the shape of a tornado. In actuality, an alveolar cleft is not limited to alveolar bone but extends to the maxilla. The goals of alveolar cleft treatment are stabilizing the maxillary arch, separating the nasal and oral cavities, and providing bony support for both erupting teeth and the nasal base via the piriform aperture. The various treatment options for alveolar cleft include gingivoperiosteoplasty (GPP), alveolar bone grafting (ABG), and alveolar distraction, and there are controversial issues regarding cleft care. In 1967, Skoog [3] reported GPP, in which periosteal continuity is constructed at the alveolar cleft during primary cleft lip repair. Additionally, he found a case of complete alveolar bone regeneration during palatoplasty. Therefore, GPP was called “boneless bone grafting.” However, some authors have reported adverse effects on facial growth and a low success rate for GPP compared with secondary ABG [4,5]. Secondary ABG has become the gold standard among the treatment options for alveolar cleft [6]. Secondary ABG is performed during the period of mixed dentition using autologous bone from various donor sites. There are several issues relevant to maximizing the success of secondary ABG, such as the surgical timing, donor site, and surgical technique. In this study, we reviewed important concepts regarding secondary ABG, including surgical timing, donor sites, and evaluation methods.
SURGICAL TIMING
ABG has traditionally been classified as primary ABG (around 2 years), early secondary ABG (2–5 years), secondary ABG (6–12 years), and tertiary ABG (>12 years) according to chronological age [7,8]. By dental age, ABG can be divided into primary ABG (deciduous dentition), secondary ABG (mixed dentition), and tertiary ABG (permanent dentition). Because chronological age and dental age are not exactly matched, the surgical timing is determined differently for each patient.
Primary ABG is usually performed during the period of primary dentition using costal bone grafts before the age of 2 years [9,10]. Primary ABG was the main surgical procedure until Boyne and Sands [11] reported secondary ABG in 1972. They recognized that primary ABG was a time-consuming procedure for young children and that it restricted maxillary growth. Therefore, secondary ABG with iliac cancellous bone was performed in 9- to 11-year-old patients, and successful regeneration of the alveolar bridge was reported in all patients. Indeed, primary ABG demonstrated unfavorable bone survival of less than 50% of the interdental height in 70%, 59%, and 39% of cases in a previous investigation [12-14]. Although Brattstrom and McWilliam [12] reported only a 39% failure rate, 24% of cases required regrafting due to total bone loss. In view of maxillary growth, some authors reported that primary ABS showed no significant difference compared with no grafting on maxillary cephalometric analysis [15-18]. However, the majority of papers have reported that primary ABG results in restricted maxillary growth, with a smaller sella-nasion-A point angle and decreased alveolar height [19-27]. Therefore, compared with secondary ABG, primary ABG is currently not recommended due to the inhibition of maxillary growth and low success rate.
Since Boyne and Sands [11] reported successful results of secondary ABG performed during the mixed dentition period between 9 and 11 years of age, it has been the optimal method for alveolar cleft reconstruction. Traditionally, secondary ABG is performed before canine eruption to facilitate canine eruption into the grafted bone [28]. In 1982, El Deeb et al. [29] performed secondary ABG during the period of mixed dentition and classified canine root development. This study concluded that the result of canine eruption was most favorable from 1/4 canine root development (root length less than crown height on radiography) to 1/2 canine root development (root length equal to crown height on radiography), when the patient was 9–12 years old. Bergland et al. [30] also advocated an ideal timing of 1/2 to 2/3 canine root development for secondary ABG. When secondary ABG was performed after canine eruption (as opposed to before canine eruption), patients exhibited accelerated resorption of the canine root and received treatment with more prosthetics [31].
Traditionally, the canine adjacent to the cleft was the key tooth used to determine the timing of secondary ABG. However, after the introduction of early secondary ABG, bone grafting was performed before lateral incisor eruption. In 2007, Ozawa et al. [32] reported that patients with the germ of the lateral incisor who underwent secondary ABG before lateral incisor eruption demonstrated less graft resorption. In particular, the volume of the migrated lateral incisor root was negatively correlated with bone graft resorption. In other words, the migrated root of the lateral incisor prevented disuse atrophy of the grafted bone and decreased resorption of the bone graft. Therefore, ABG at 5 to 7 years of age could save the lateral incisor and result in symmetrical and healthy occlusion in patients with the lateral incisor germ. The researchers concluded that secondary ABG should be performed at an earlier age in patients with than without the lateral incisor germ. In 2016, Dissaux et al. [28] compared the results of bone survival and tooth eruption after ABG performed between 5 and 10 years of age. The researchers also reported that early secondary ABG before lateral incisor eruption (at 5 years of age) resulted in greater bone survival on three-dimensional (3D) volumetric analyses than that before canine eruption (at 10 years of age). They concluded that early secondary ABG yielded a higher success rate than traditional secondary ABG, although the impact of early secondary ABG on facial growth was still questionable. In 2018, Doucet et al. [33] found that early secondary ABG at approximately 6 years of age did not influence midfacial growth as assessed by the sella-nasion-A point angle compared with traditional secondary ABG at 9 to 11 years of age. Therefore, the lateral incisor could be an additional key tooth for determining the timing of secondary ABG. In patients with the lateral incisor germ, early secondary ABG facilitates bone survival and healthy occlusion. However, to prevent bone resorption due to disuse, early secondary ABG should not be performed until canine eruption in patients without lateral incisor germ. A survey of 53 American Cleft Palate-Craniofacial Association-approved multidisciplinary cleft teams revealed that secondary ABG at 6 to 8 years of age (early secondary ABG) and at 8 to 13 years of age (traditional secondary ABG) were similarly performed in 47% and 49% of the cleft teams, respectively [34].
GRAFT MATERIALS
Iliac bone
Iliac cancellous bone is considered the gold standard graft material for secondary ABG because of the abundant cancellous bone, easy access, and possibility of a two-team approach. Cancellous bone contains a large number of osteogenic cells and exhibits fast revascularization, in contrast to cortical bone, which maintains volume by creeping substitution [35,36]. In 1991, Kortebein et al. [37] reported that secondary ABG with iliac cancellous bone showed a higher success rate (89.8%) than that with calvarial corticocancellous bone (63%). Disadvantages of iliac bone that may limit its use include postoperative pain and residual scarring [38]. However, postoperative pain was found to be frequently overestimated and easily controlled by analgesics [39]. In particular, harvesting bone via a minimally invasive technique with a trephine instead of via an open technique could reduce postoperative pain.
Calvarial bone
Calvarial bone is an intuitionally attractive graft material because of the shared embryologic origin of membranous and alveolar bone and closeness to the recipient site; additionally, harvesting involves less pain, and the scar can be hidden by hair [37,40]. Zins and Whitaker [41] reported that membranous bone could maintain its volume with less resorption than endochondral bone when grafted in the craniofacial region. However, the cortical bone block was grafted in a pattern different from that in ABG, as grafting was performed by creating an inlay pattern with bone particles. Indeed, Rosenthal and Buchman [42] reported that the volume of grafted bone depends on the characteristics of that bone (cancellous vs. cortical), regardless of its embryologic origin. Although many papers reported discouraging results for calvarial bone graft survival compared with iliac bone graft survival, the researchers used a significant proportion of cortical bone grafts [37,40]. Because some authors have still reported favorable results using calvarial bone in secondary ABG, selection of the donor site may be influenced by the surgeon’s preference [43,44].
Tibial bone
Although the tibial bone is a well-established donor site for bone grafts in orthopedic surgery, most experience with tibial grafts has been in adults, mainly for trauma [45]. Few authors have reported favorable results of ABG using tibial bone [46,47]. Additionally, there is the risk of damage to growing epiphyseal cartilage during the harvest of bone grafts.
Bone substitutes
Allogenic bone grafts are an attractive option for secondary ABG because of their lack of donor site morbidity, reduced pain, and large volume [38]. The first allogenic bone grafting procedure for alveolar cleft repair was performed by Kraut in 1987 [48]. He performed secondary ABG using allogenic freeze-dried bone and obtained successful bone regeneration and canine eruption in five cases. Recently, a double-blind randomized control study compared iliac cancellous bone grafts with bovine-derived demineralized bone matrix (DBM) in secondary ABG [49]. In this study, there was no significant difference between DBM and iliac cancellous bone grafts on volumetric analysis at the mean 63-month follow-up. Although the cost of surgery was higher in the DBM group, there was no donor site morbidity. Therefore, they concluded that DBM could be used as an analog to autologous cancellous bone. However, this study enrolled a small number of patients, with only 10 patients in each group. It is clear that DBM is a potential graft material for secondary ABG. However, further research in more patients will be needed to clarify its efficacy and safety in secondary ABG.
Recombinant human bone morphogenetic protein (rhBMP)-2 is also a potential alternative to cancellous iliac bone grafts. rhBMP-2 was approved by the U.S. Food and Drug Administration as a bone graft substitute for maxillary sinus augmentation [50]. In 2017, Hammoudeh et al. [50] reported similar rates of regrafting and canine eruption with rhBMP-2/DBM and iliac cancellous bone grafts in secondary ABG. Alonso et al. [51] analyzed nasal symmetry after secondary ABG with iliac cancellous bone versus an rhBMP-2/collagen sponge. They reported a similar effect on nasal symmetry in both groups. A common complication of secondary ABG with rhBMP-2 is local gingival edema [52]. Because there are still insufficient data on the long-term efficacy and safety of rhBMP-2 in children, rhBMP-2 should be applied carefully in secondary ABG.
EVALUATION METHODS
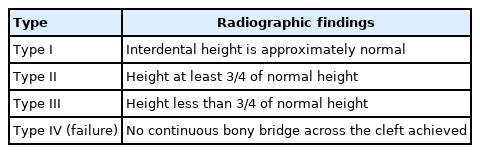
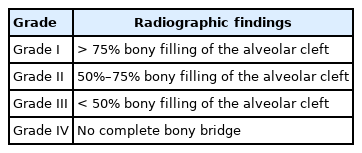
Dental radiography is the most widely accepted method for evaluating the results of secondary ABG [53]. There are several scoring systems for plain radiographs, such as the Bergland [30], Kindelan [54], Enemark [55], Abyholm [56], and Chelsea [57] scoring systems. In a 2018 survey of cleft team coordinators in the UK and Ireland, the majority of centers used the Kindelan (63%, 24/51 responders) and Bergland scoring systems (27%, 10/51 responders) [58]. The Bergland and Kindelan scoring systems consist of semiquantitative analyses based on dental radiographs, mostly of the occlusal view. The Bergland and Kindelan scoring systems are presented in Tables 1 and 2, respectively. In these systems, success is considered in the case of type I or II findings. However, two-dimensional (2D) analysis cannot reflect the actual bone thickness or volume. Han et al. [44] reported a correlation of the alveolar height determined by plain radiography and computed tomography. However, the alveolar thickness was underestimated on computed tomography compared to plain radiography. Therefore, 3D analysis using conebeam computed tomography (CBCT) has gained attention because it involves less radiation multidirectional computed tomography and offers the possibility of volumetric analysis [59]. However, there are diverse methods to analyze volume which obscure criteria for success. Therefore, future studies to reach a consensus on a CBCT-based grading system will be necessary.
CONCLUSION
Secondary ABG is a well-established treatment for alveolar cleft. The timing of surgery for alveolar cleft is traditionally in the period of mixed dentition before canine eruption. Recently, early secondary ABG was introduced by some published studies in which the procedure was performed at approximately 6 years of age to save the lateral incisor. The standard evaluation method for analyzing bone graft survival is 2D analysis by dental radiography. However, 2D analysis cannot be used to evaluate bone volume or thickness. Although 3D analysis by CBCT has been emphasized by recent papers, a standard evaluation method based on CBCT is still lacking.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contribution
Conceptualization: Woonhyeok Jeong. Data curation: Woonhyeok Jeong. Funding acquisition: Woonhyeok Jeong. Methodology: Woonhyeok Jeong. Writing - original draft: Junhyung Kim. Writing - review & editing: Woonhyeok Jeong. Supervision: Junhyung Kim, Woonhyeok Jeong. Validation: Woonhyeok Jeong.
Abbreviations
ABG
alveolar bone grafting
CBCT
cone-beam computed tomography
DBM
demineralized bone matrix
GPP
gingivoperiosteoplasty
rhBMP
recombinant human bone morphogenetic protein
2D
two dimensional
3D
three dimensional