Perforating patterns of cutaneous perforator vessels in anterolateral thigh flaps for head and neck reconstruction and clinical outcomes
Article information
Abstract
Background
Anterolateral thigh (ALT) flaps are versatile soft tissue flaps that have become the standard soft-tissue flaps used for head and neck reconstruction. They provide a long vascular pedicle, constant vessel diameter, abundant soft tissue coverage, and minimal donor site morbidity. The ALT flap was initially designed on the basis of a septocutaneous (SC) perforator. However, more recent research has shown that a substantial number of ALT flaps are now based on musculocutaneous (MC) perforators, and the ratio between MC and SC perforators varies among studies. In this study, we analyzed the perforating pattern of ALT flaps along with their clinical outcomes during head and neck reconstruction in the Korean population.
Methods
From October 2016 to July 2020, 68 patients who had undergone an ALT flap procedure for head and neck reconstruction were enrolled retrospectively. The perforating pattern of the cutaneous perforator vessel (MC perforator/SC perforator/oblique branch), pedicle length, and flap size were analyzed intraoperatively. Patient demographics and flap necrosis rates were also calculated.
Results
The highest number of cutaneous perforator vessels supplying the ALT flap were the MC perforators (87%). The proportion of MC perforators was significantly higher than that of the SC perforators and oblique branches. Flap necrosis occurred in seven cases (11.86%); sex, hypertension, diabetes mellitus, coronary artery disease, perforator course, and history of radiotherapy did not significantly affect flap necrosis.
Conclusion
The ALT free flap procedure remains popular for reconstruction of the head and neck. In this study, we observed that the majority of cutaneous vessels supplying the flaps were MC perforators (87%). When using the MC perforator during flap elevation, careful dissection of the perforator is required to achieve successful ALT flaps because intramuscular dissection is difficult. Perforator pattern and history of radiotherapy did not affect flap necrosis.
INTRODUCTION
Head and neck cancers are the sixth most common cause of malignancy, and excision of tumors in the head and neck often cause aesthetic and functional problems [1]. With recent developments in microsurgery, free-flap reconstruction has become first-line treatment for head and neck reconstruction, as it can cover both aesthetic and functional aspects [2].
The anterolateral thigh (ALT) flap procedure was first described by Baek in 1983 [3] and again by Song et al. in 1984 [4]. Its numerous advantages include a long vascular pedicle, good vessel diameter, availability of different tissues with large amounts of skin, and minimal morbidity at the flap donor site [5-7]. The flap can also be designed as myocutaneous, fasciocutaneous, adipofascial, and suprafascial [5,8]. Thus, ALT has a suitable surface for head and neck reconstruction [6,9,10]. Despite this versatility, the ALT flap has not been widely used in the past, as the majority of cutaneous perforators supplying the flap are musculocutaneous (MC) vessels, causing intramuscular dissection to sometimes become technically difficult and unsafe [11,12]. However, with the increasing use of perforator flaps and the development of intramuscular perforator dissection techniques, use of ALT flaps is on the rise [5,6]. According to one study on perforator patterns, MC perforators accounted for a higher percentage than septocutaneous (SC) perforators, however the percentage varies from study to study (Table 1). In comparison to the research on perforators of the ALT in other countries, there is a lack of studies in Korea. Thus, this study aimed to analyze the cutaneous perforators of ALT flaps along with their clinical outcomes.
METHODS
Following approval from the institutional review board, patients who had undergone ALT free flap procedures for defects after head and neck cancer surgery between October 2016 and July 2020 at our hospital were included. Medical records were retrospectively reviewed.
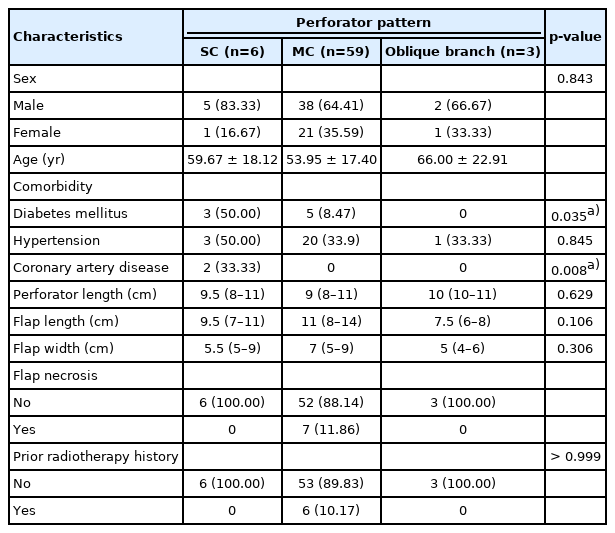
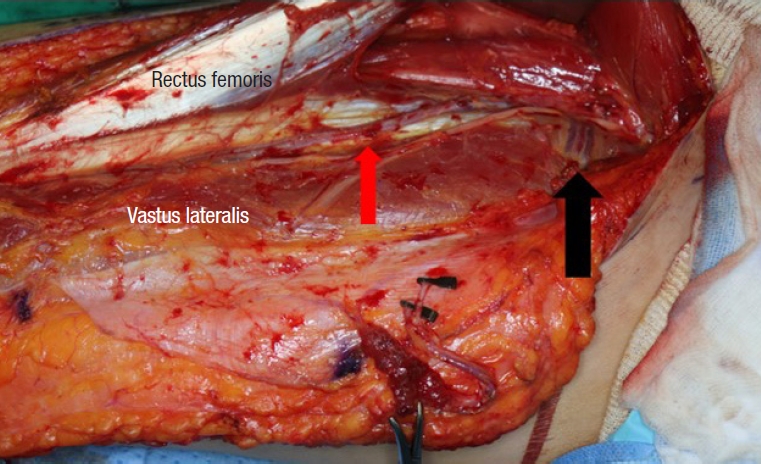
During ALT flap elevation, the cutaneous perforator was located by opening the intramuscular septum between the rectus femoris and vastus lateralis muscles. When we opened the septum, the descending branch of the lateral circumflex femoral artery (LFCA) was able to be identified. The cutaneous perforators commonly lying within this septum are either SC perforators (Fig. 1) or MC perforators (Fig. 2). Where the oblique branch, which runs laterally to the descending branch, was the main perforator of the ALT flap, the oblique branch was used (Fig. 3). We recorded whether the SC perforator, MC perforator, or oblique branch was used as a cutaneous perforator; and measured perforator length, flap size, and clinical outcome.
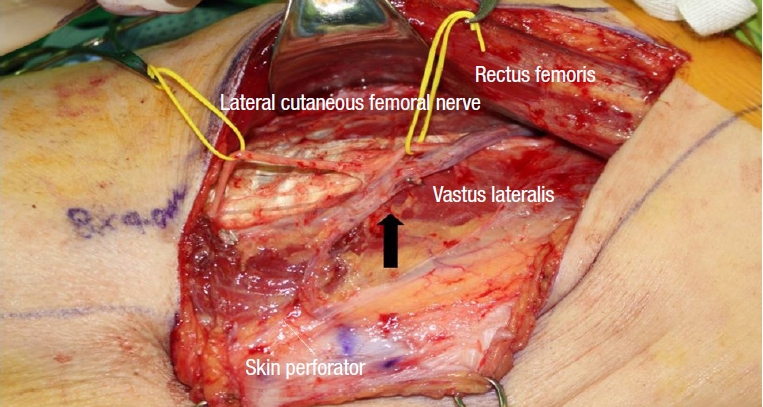
Pedicle is visible in the intramuscular septum between rectus femoris and vastus lateralis. Black arrow indicates septocutaneous branch of lateral circumflex femoral artery.

Black arrow indicates musculocutaneous branch of the lateral circumflex femoral artery traversing the vastus lateralis muscle.
Black arrow indicates oblique branch of lateral circumflex femoral artery. This runs between the descending and the transverse branches of the lateral circumflex femoral artery. Red arrow indicates descending branch of lateral circumflex femoral artery.
All analyses were performed using SAS software (SAS Institution Inc., Cary, NC, USA). We compared and analyzed each variable using an SC perforator, MC perforator, and oblique branch. If continuous variables followed normality through the Shapiro-Wilk test, they were analyzed by one-way analysis of variance and independent t-test; if they did not follow normality, the Kruskal-Wallis and Wilcoxon rank-sum tests were used. Categorical variables were analyzed using the chi-square test and Fisher effect tests. Continuous variables are denoted as mean±standard deviation [SD] if they followed normality; median (interquartile range, IQR) if they do not follow normality, and categorical variables as counts (%). The chi-square test was used to determine whether there was a significant difference in the ratio of the perforator pattern between the three groups (SC pattern, MC pattern, and oblique branch). Since multi-competition between the three groups was implemented, the significant p-value was 0.017.
RESULTS
Patient demographics and perforator characteristic
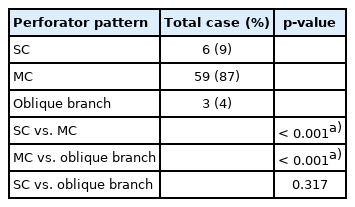
A total of 68 patients were retrospectively identified as eligible for inclusion in this study. The clinical characteristics of the study population are summarized in Table 2. Of the 68 patients, six flaps (9%) had an SC perforator pattern, 59 flaps (87%) had an MC perforator pattern, and three flaps (4%) had an oblique branch pattern. The mean age was 59.67 years (SD, 18.12 years) in the SC group, 53.95 years (SD, 17.40 years) in the MC group, and 66.00 years (SD, 22.91 years) in the oblique group. Diabetes mellitus (DM) occurred in eight cases, hypertension (HTN) in 24 cases, and coronary artery disease in two cases. Six patients had received prior radiotherapy, and were in the MC group. The median pedicle length of the SC was 9.5 cm (IQR, 8–11 cm), that of the MC was 9 cm (IQR, 8–11 cm), and that of the oblique branches was 10 cm (IQR, 10–11 cm) for (Table 2). The median flap length of the SC was 9.5 cm (IQR, 7–11 cm), that of the MC was 11 cm (IQR, 8–14 cm), and that of the oblique branches was 7.5 cm (IQR, 6–8 cm), respectively. The median flap width of the SC was 5.5 cm (IQR, 5–9 cm), that of the MC was 7 cm (IQR, 5–9 cm), and that of the oblique branch was 5 cm (IQR, 4–6 cm). In the case of flap necrosis, there were seven cases which occurred exclusively in the MC group. There was a significant difference in the incidence of DM and HTN among the three groups. No other factors showed a significant difference between the three groups (Table 2).
Comparison between the perforator pattern according to total cases
The difference in perforator pattern ratio was significantly higher in the MC group when comparing SC and MC (p<0.001), and significantly higher in the MC group when comparing MC and oblique (p<0.001). However, there was no significant difference when comparing SC and oblique branches (p=0.317). In other words, the number of MCs was significantly higher than that of SC or oblique (Table 3).
Variables regarding flap failure
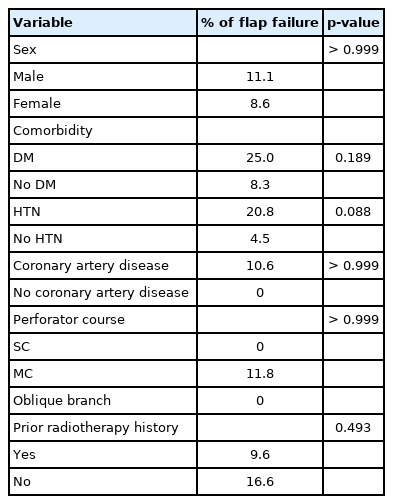
Sex, DM, HTN, and coronary artery disease had no significant effect on flap survival (Table 4). There was no significant difference in flap survival depending on whether the perforator was SC, MC, or oblique branch. Prior radiotherapy also had no significant effect on the flap survival.
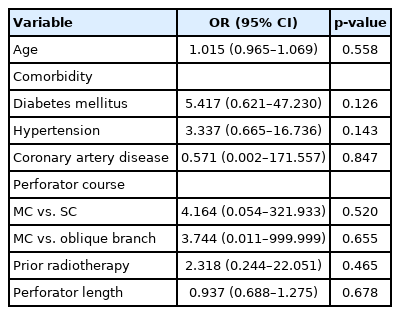
We analyzed whether there were factors affecting flap failure using the univariable logistic regression analysis, and there were no factors that significantly affected flap failure (Table 5). Using firth multivariable logistic regression analysis, other covariates were corrected, and there were no factors that significantly affected flap failure (Table 6).
DISCUSSION
The ALT flap has the advantages of long pedicle length, adequate size, moderate thickness, low morbidity of the flap donor, possibility of chimeric flap, division of flap based on perforators, harvest using a large skin paddle, and the ability to preserve the lateral femoral cutaneous nerve along with flap sensation [9,19-22]. Despite these advantages, the ALT free flap has not gained widespread use as dissection of the vascular pedicle may be challenging due to anatomical variation. In particular, cutaneous perforators are commonly MC vessels arising from the descending branch of the LFCA and penetrating the vastus lateralis muscle, making it difficult to harvest the flap. With the development of techniques for intramuscular dissection and research on perforators, ALT has become widely used [5,6]. However, research on perforator patterns in the Korean population is lacking. The aim of this study was to discuss perforator pattern, length, size, and flap failure rate in the Korean population for head and neck reconstruction.
In general, the cutaneous perforator of the ALT flap arises from the descending branch of the LFCA. This descending branch runs through the intermuscular space between the rectus femoris and vastus lateralis muscles. Cutaneous perforators are classified into three types: SC perforators, MC perforators, and oblique branches [23]. The SC perforator vessel runs through the intermuscular septum between the rectus femoris and vastus lateralis. This perforator can be easily mobilized and flap harvest can be expediently completed [23]. However, dissection of the MC perforator, which penetrates the vastus lateralis muscle, is notorious as dissection begins with the unroofing of the muscle that covers the perforator and its surrounding loose areolar tissue [24]. When present, the oblique branch runs between the descending and transverse branches of the lateral circumflex artery, and may originate from the descending branch, transverse branch, LFCA, profunda femoris, or directly from the femoral artery. It has been found to be present in 35% of patients [25].
Kimata et al. [13] have demonstrated that in Japan, MC perforators are more common than SC perforators (81.9% vs. 18.1%). Similarly, Xu et al. [14] have reported that MC perforators were found in 60% of patients studied in China, and SC perforators in 40%. In the United States, Shimizu et al. [15] have reported that of 42 perforators studied, 51% were MC and 49% were SC perforators; and Seth et al. [17] reported 66.1% of MC perforators. In Taiwan, Shieh et al. [16] reported 83.8% MC perforators and 16.2% SC perforators, and Wei et al. [5] reported that MC perforators account for 87.1% of perforators. In India, Seetharaman et al. [18] have found 61.8% of MC perforators in India. In a cadaveric study, Choi et al. [24] reported that MC perforators accounted for 82.5% of 160 perforators, while SC perforators accounted for 17.5% (Table 1).
In our study, we observed the MC perforator in 87%, the SC perforator in 9%, and the oblique branch in 4% of patients. Comparison among the three groups showed that the ratio of the MC was significantly higher than that of the SC or oblique branches (Table 3). Our finding that the proportion of MC was higher than that of SC and oblique branches with result corresponds to the existing studies. In a cadaveric study by Choi et al. [24], the proportion of MC in the Korean population was 87%, which is specifically consistent with our study. There is no established theory as to why MC group is more than SC group. Palackic et al. [26] reported on the anatomy of the LCFA. They classified LCFA into four groups (A, B, C, and D) according to the numbers of the branches. group A included a total number of three branches, group B included four, group C included five and group D included only two branches. Among them, in the case of groups A, B, and C, which accounts for about 94.2%, there is a branch that pierces vastus lateralis, but in the case of group D, which accounts for about 5.8%, there is no branch that pierces vastus lateralis. Since most of the LFCA branches pierces the vastus lateralis, it can be estimated that the majority of cutaneous perforators from LFCA are MC group.
The mean pedicle length of the SC group was 9.5 cm (IQR, 8–11 cm), that of the MC group was 9 cm (IQR, 8–11 cm), and that of the oblique branch group was 10 cm (IQR, 10–11 cm), and there was no significant difference between the three groups (Table 2). According to Deng et al. [27], the diameter of the oblique branch is generally small, and the vascular pedicle is also shorter than the average ALT flap; however, there was no significant difference in our results (Table 2).
Seven cases of flap necrosis occurred in the MC group only (Table 2). Of the seven cases, total flap necrosis occurred in two cases, and was caused by poor flap vascularization, of two cases, one case was re-operated to the radial forearm free flap after flap detachment, and in the other case, only flap detachment was performed. Partial flap necrosis occurred in five cases. Of the five cases, two were managed by re-anastomosis, one by flap detachment and re-operation to the latissimus dorsi free flap, and two by salvage. However, there were no significant differences among the three groups (Table 2). There was no difference in the flap necrosis results when using the SC, MC, and oblique branches. Generally, it is more difficult to elevate the ALT flap using MC, but there is no difference in the flap survival rate. This is thought to be due to the increase in the use of perforator flaps and technological development of intramuscular dissection. Comparing oblique branches with SC and MC, Deng et al. [27] reported that the flap survival rate when using the oblique branch is similar to that when using SC or MC. There were no significant factors affecting flap survival among sex, comorbidity (DM, HTN, and coronary artery disease), main perforator of flap, and prior radiotherapy. There is debate as to whether prior radiotherapy history affects flap survival. Tasch et al. [28] demonstrated that prior irradiation has a significant effect on total and partial free flap failure. However, Kim et al. [2] have reported that prior radiotherapy or neck dissection did not affect the success rate of the flap even when a free flap was used for recurrent head and neck cancer. In addition, Suh et al. [29] have reported that prior radiotherapy, sex, and comorbidities did not affect the success rate of the flap. Simpson et al. [30] argued that factors other than the surgeon’s surgical experience did not significantly affect the flap success rate. Our study also found that a history of pre-radiotherapy did not affect flap survival.
Our study had some limitations. First, this study represents a small cohort of patients. Therefore, a prospective or matched cohort study may be beneficial for validating these results. It was found that there was no significant difference in flap necrosis rate depending on the pedicle type, but it is difficult to conclude affirmatively because it is a small size sample and has a large sample difference between each group. Further research is needed to use a large sample size and reduce the sample difference between each group. Second, unlike cadaveric studies, not all anatomies were found intraoperatively. Therefore, additional research is needed to determine the exact proportion of oblique branches. Lastly, we found more oblique branches intraoperatively, but we did not use an oblique branch because we could use the SC or MC as the main perforator. In other words, it cannot be said that only 4% of patients had oblique branches, and research on this is needed. Although there are such limitations, it is significant in that it analyzes perforator patterns and clinical outcomes.
In conclusion, most perforator patterns were MC perforators (87%), and SC perforators accounted for only 9%. MC perforators are almost 10 times more common than SC perforators in Korea. Although there was no difference in flap survival, since most perforators are of the MC type, it is necessary to carefully dissect the perforator. The perforator pattern used to perform the ALT flap did not affect flap survival rate. In addition, an oblique branch may be useful in the absence of an appropriate cutaneous perforator.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported. The informed consent was waived.
Ethical approval
The study was approved by the Institutional Review Board of Severance Hospital (IRB No. 4-2022-0039) and performed in accordance with the principles of the Declaration of Helsinki. The informed consent was waived because this design is a retrospective study.
Patient consent
The patients provided written informed consent for the publication and the use of their images.
Author contribution
Conceptualization: Jong Won Hong, In-Sik Yun. Data curation: Sang Soo Lee. Formal analysis: In-Sik Yun. Methodology: InSik Yun. Visualization: Sang Soo Lee. Writing - original draft: Sang Soo Lee. Writing - review & editing: Sang Soo Lee. Supervision: Won Jae Lee.
Abbreviations
ALT
anterolateral thigh
CI
confidence interval
DM
diabetes mellitus
HTN
hypertension
LFCA
lateral circumflex femoral artery
MC
musculocutaneous
OR
odds ratio
SC
septocutaneous
SD
standard deviation