Scalp reconstruction using the reverse temporalis muscle flap: a case report
Article information
Abstract
The scalp is the thickest skin in the body and protects the intracranial structures. The coverage of a large scalp defect is a difficult surgical procedure, the full details of which must be considered prior to the procedure, such as defect size and depth, and various factors related to the patient’s general condition. Although a free flap is the recommended surgical procedure to cover large scalp defects, it is a high-risk operation that is not appropriate for all patients. As such, other surgical options must be explored. We present the case of a patient with an ulcer on the scalp after wide excision and split-thickness skin graft for squamous cell cancer. We successfully performed a reverse temporalis muscle flap for this patient.
INTRODUCTION
Scalp reconstruction is difficult even for the most experienced plastic surgeons. The convex shape, protection of intracranial structures, and cosmetic outcome are essential considerations.
There are various surgical techniques including skin graft, local flap, and free flap. Previous studies about scalp reconstruction have proposed a reconstructive decision-making algorithm according to the location, size, and depth [1,2]. Free flap coverage can be considered the first-line surgical option as it provides good vascularization and appropriate soft tissue bulk to the affected area. However, a free flap is a high-risk procedure for older patients with underlying medical conditions. Therefore, alternative surgical approaches are often considered. Here, we introduce a case of successful scalp reconstruction using a reverse temporalis muscle flap (RTMF).
CASE REPORT
A 77-year-old female patient presented to the outpatient clinic with a chronic ulcer on her left temporo-occipital scalp. She had undergone a wide excision and split-thickness skin graft (STSG) for squamous cell carcinoma of the scalp 18 years ago (Fig. 1). During the initial surgery, squamous cell carcinoma had been found to invade the calvarial cortical bone partially. A cortical ostectomy was performed, and an STSG (10×12 cm) was performed to cover the defect. Afterward, a 5×5 cm ulcer developed with bony involvement due to partial STSG loss. Computed tomography showed a defect in the outer cortex of the cranium (Fig. 2).

Preoperative computed tomography images. Soft tissue defect and thinning parietal bone are seen on the left temporoparietal scalp. (A) Coronal and (B) Sagittal views.
To determine the regression of cancer, a biopsy was performed. The pathology report revealed an epidermal cyst. The main contributing factor of poor wound healing was deemed to be close proximity of the bone and skin. Therefore, we decided to reconstruct the defect in order to provide adequate soft tissue between the bone and skin.
Free flap coverage would have been a viable surgical technique. However, free flap coverage was deemed inappropriate due to an expected prolonged operative time given the patient’s advanced age, underlying hypertension, and severe malnutrition (height 143 cm and weight 38 kg). Subsequently, alternative surgical techniques were explored, and we decided upon an RTMF due to its superior volume coverage, as well as the advantageous size and location of the defect for an RTMF.
The temporalis muscle is supplied by three primary arteries: anterior deep temporal artery in anterior area, posterior deep temporal artery in middle area and middle temporal artery in posterior area. Also, venous network correlates well with the arterial network. The capillaries formed a dense, interlacing network with orientation along the muscle fibers [3]. On the other hand, the superficial temporal artery provides blood supply to the superficial temporal fascia (temporoparietal fascia).
The dense plexus of the vascular zone extends from the superficial temporal line to approximately 1.8 cm below the superficial temporal line. This zone creates the capillary anastomosis network between the temporalis muscle and superficial temporal fascia [4]. The key focus during dissection is to keep the dense plexus of the vascular zone intact while elevating the temporalis muscle flap from the superficial temporal fascia. During flap elevation, the deep temporal artery is ligated, but the vascular supply of the muscle is maintained thanks to an intact capillary anastomosis network.
The plan was to provide coverage only to the main ulcer area (5×5 cm) of the previous STSG site (10×12 cm). Therefore, debridement over the main ulcer area was performed. Flap elevation was initiated from the zygomatic arch area. The loose areolar tissue between superficial temporal fascia and deep fascia was dissected to separate the superficial layer supplied by the superficial temporal artery and the deep layer supplied by the deep temporal artery. Dissection was performed up to the dense plexus of the vascular zone. After completing the dissection, the vascular zone was sutured using an absorbable suture (5-0 Vicryl) to ensure that the temporalis muscle and superficial temporal fascia remained intact and inseparable in the vascular zone.
The elevated flap was cut off at the coronoid process of the mandible. The flap was then turned over and transpositioned towards the direction of the defect, and the deep temporal fascia was adjusted towards the skull surface. After insetting the flap, the distal tip of the flap did not completely cover a small portion of the defect (1×4 cm) and therefore Matriderm (Dr. Suwelack, Billerbeck, Germany) was applied to the site. Lastly, an STSG was harvested from the left thigh to provide coverage over the flap. This approach enabled us to achieve soft-tissue coverage as well as volume restoration in comparison with the previous STSG. The surgical procedure is shown in a simple schematic diagram (Fig. 3).
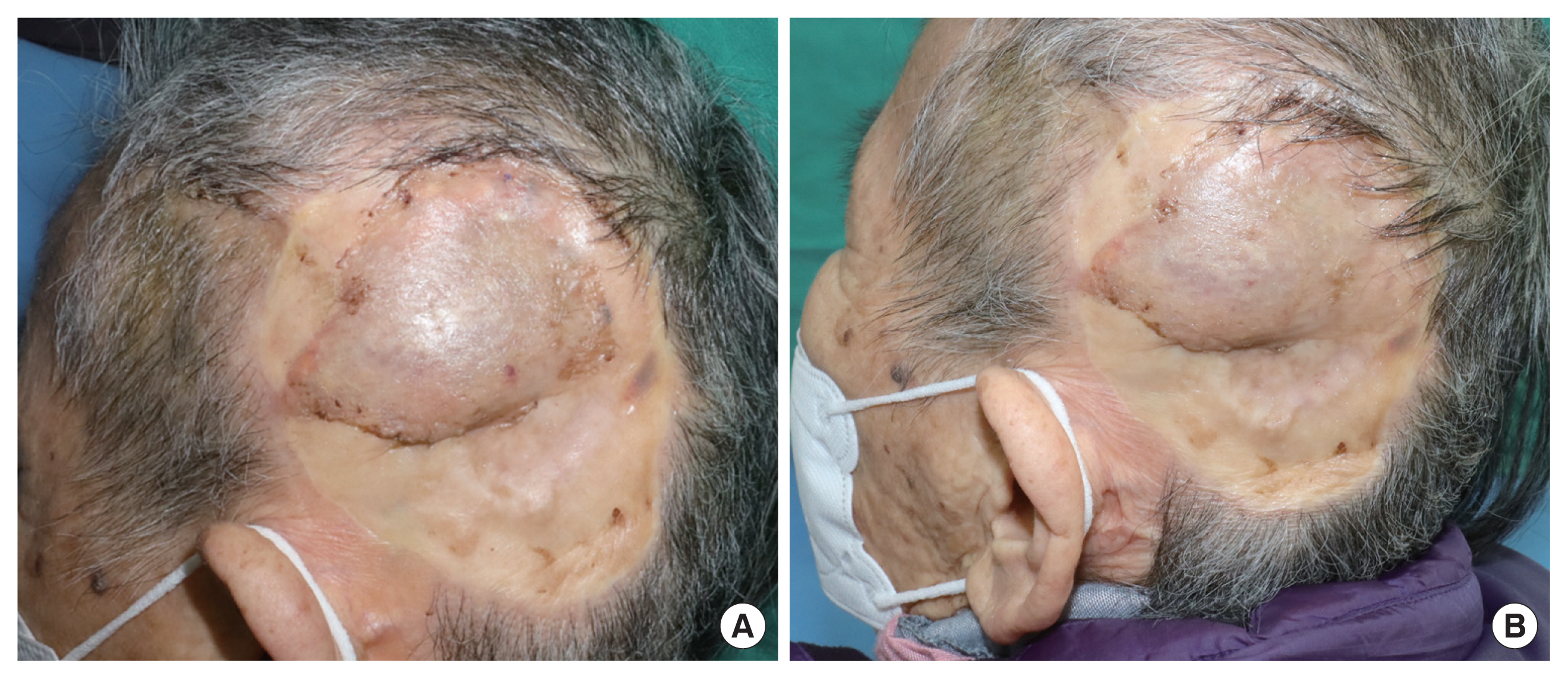
The total surgery time was 3 hours and 25 minutes. Two months after surgery, the wound has healed well (Fig. 4). The reconstruction was successful without any significant complications. The facial nerve branches were preserved well, and functional conservation was confirmed in her facial expression. When she furrowed her eyebrows, closed eyes and smiled lifting the lip corners, symmetric facial expression was demonstrated. Cautious dissection and turn-over procedures posterior to Pitanguy’s line (PL) prevented unintended injuries of facial nerve branches. The bulky tissue above the skull effectively provided a protective effect.
DISCUSSION
Various approaches can be considered for scalp reconstruction based on the size, volume, and location of the defect. STSG has the advantages of simplicity and good healing. However, it is susceptible to trauma or infection due to its low durability. Local flap using scalp tissue may provide hair-bearing skin but only provides coverage for a small defect. In the reconstruction of the head and neck area including the scalp, the free flap is a good surgical method for a patient who is in good general condition [5]. Free flap coverage can provide adequately bulky tissue with vascularity, and is therefore appropriate for the reconstruction of large bulky tissue defects. However, there is a high risk of potential complications due to flap loss, prolonged surgical time, and donor morbidity. Chang et al. [6] reported a complication rate of 31% in cases in which patients received free flap coverage for the defect after an initial cranial base tumor surgery. Another study by Newman et al. [7] reported a similar complication rate of 28.6%. These complications included flap loss, hematoma, and infection.
Kim and Park [8] first used RTMF to successfully provide coverage for large anterior cranial base defects in 1995. We utilized this approach to cover a large volume defect on the temporo-occipital area. Cheung [3] presented the vascular anatomy of temporalis muscle in detail. With regard to the study of coronal plane, arteries were distributed mainly on the lateral and medial portions. The anterior deep temporal artery and posterior deep temporal artery, the branches of maxillary artery were discovered on the medial portion. Two vascular sources were observed on the lateral portion, namely the superficial temporal artery and the maxillary artery. Most vascular supplies depended on the middle temporal artery, one of the branches of the superficial temporal artery.
Newman et al. [7] suggested a scalp reconstruction algorithm according to wound size and local tissue quality. If the wound is small and surrounding tissue is of good quality, STSG or local flap are recommended. If not, a free flap is recommended. According to this algorithm, the reconstructive site described in this case, approximately 5×5 cm in size and with poor local tissue quality, would have required a free flap [7]. Hanasono et al. [9] reported that the average surgical time for a free flap was 11.2 hours, whereas the surgery in this case was only 3.5 hours long. The advantage of our surgical approach includes a short operation time due to an absence of microsurgery.
The operator must pay close attention to prevent any damage to the facial nerve while dissecting the temporalis muscle. An imaginary line called PL uses surface landmarks by locating the line that runs from 0.5 cm below the tragus to 1.5 cm above the lateral eyebrow. A cautious dissection keeping distance from the PL minimizes the risk of injury to the frontotemporal branch of the facial nerve [10]. In this case, dissection was performed on the scalp 1 cm posterior to PL during the flap elevation to minimize the risks of nerve injury.
Traditional temporalis muscle flap could also be a surgical option. The flap is well known as a cosmetic and functionally beneficial surgical approach used for the reconstruction of the orbit and maxilla [11]. However, the pedicle length is relatively short. In addition, the muscle volume at the distal end of the flap that could possibly be used for defect coverage was of inadequate bulk. As a result, it was difficult to obtain a sufficient arc of rotation of the flap to cover such a large defect in the temporo-occipital area.
Sternocleidomastoid (SCM) flap is one of the surgical options when performing head and neck reconstruction. In this patient, SCM flap was initially considered as a potential surgical approach based on a case report of a successful coverage of a similar defect using an SCM flap supplied by the occipital artery [12]. However, given the larger defect size and shorter rotation arc of the flap, RTMF was considered to be a safer option in this case.
Our study was limited by the finding that although the main ulcerative lesion was adequately covered using RTMF, the size of the flap was inadequate to completely cover the lower part of the previous STSG. Another limitation was our insufficient preoperative strategy to find the optimal surgical procedure. Several papers report that RTMF is versatile and reasonable, however, the donor site morbidity like weakness and depression should not be ignored. The authors could have found an easy way to cover the defect without donor site deformity and additional skin graft. Nonetheless, the problematic ulcerative lesion was restored successfully without notable events during the recovery period.
In conclusion, RTMF is an appropriate alternative option to free flaps when treating large soft tissue defects with volume loss on the scalp.
Abbreviations
PL
Pitanguy’s line
RTMF
reverse temporalis muscle flap
SCM
sternocleidomastoid
STSG
split-thickness skin graft
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was approved by the Institutional Review Board of Konkuk University Medical Center (IRB No. KUH 2022-02-055).
Patient consent
The patient provided written informed consent for the publication and the use of her images.
Author contribution
Conceptualization: Myungchul Lee. Data curation: Youngsu Na. Methodology: Myungchul Lee. Writing - original draft: Youngsu Na. Writing - review & editing: Myungchul Lee. Supervision: Myungchul Lee, Donghyeok Shin, Hyungon Choi, Jeenam Kim.