Periorbital cutaneous angiomyolipoma: a case report
Article information
Abstract
Angiomyolipomas are usually found in the kidneys of patients with tuberous sclerosis. They occur less frequently in organs such as the liver, the oral cavity, the nasal cavity, the heart, the large intestines, and the lungs. Angiomyolipomas of the skin are extremely rare, and cutaneous angiomyolipomas generally occur on the elbow, the ends of digits, the ear, and the glabella. Herein we present a rare case of angiomyolipoma occurring on the face—specifically, the right upper eyelid. We propose that upper eyelid angiomyolipoma is a hamartomatous, rather than neoplastic, lesion. Although angiomyolipoma in the periocular area is rare, it should be considered in the differential diagnosis of clinically benign masses. and regular follow-up is warranted.
INTRODUCTION
Angiomyolipoma (AML) is a benign mesenchymal hamartomatous neoplasm consisting of a variable and mixed pattern of adipose tissue, smooth muscle bundles, and abnormal blood vessels [1]. It is considered a perivascular epithelioid cell tumor (PEComa) and characteristically expresses both smooth muscle markers and melanocytic markers [2]. AML usually occurs in the kidney as a sporadic tumor or as part of the tuberous sclerosis complex [1]. However, it has also been reported in extrarenal locations, such as the liver, uterus, and other infrequent sites, such as the heart, lungs, and intestines. The liver is the second most common site other than the kidney [3].
Renal AML has small to medium-sized with blood vessels with epithelioid components but extrarenal AML has large blood vessels often without epithelioid components. In addition, renal AML shows signs of tuberous sclerosis complex up to 30% while in the extrarenal AML, patients are not afflicted with tuberous sclerosis complex. For this reason, after excision of mass, extrarenal AML have better prognosis. Moreover, renal AML is more invasive and display human melanoma black-45 (HMB-45) positivity in 95% of the patients. whereas extrarenal AML has no recurrences and lack positivity to HMB-45 [4].
Although numerous case reports have described cases of extrarenal AML, reports on AML in the craniofacial area are still rare [5]. In this case report, we present a case of extrarenal AML on the upper eyelid, which had gradually increased in size over the course of 10 years in a 43-year-old man with no underlying disease. To our knowledge, this is the first report of a cutaneous AML in the upper eyelid.
CASE REPORT
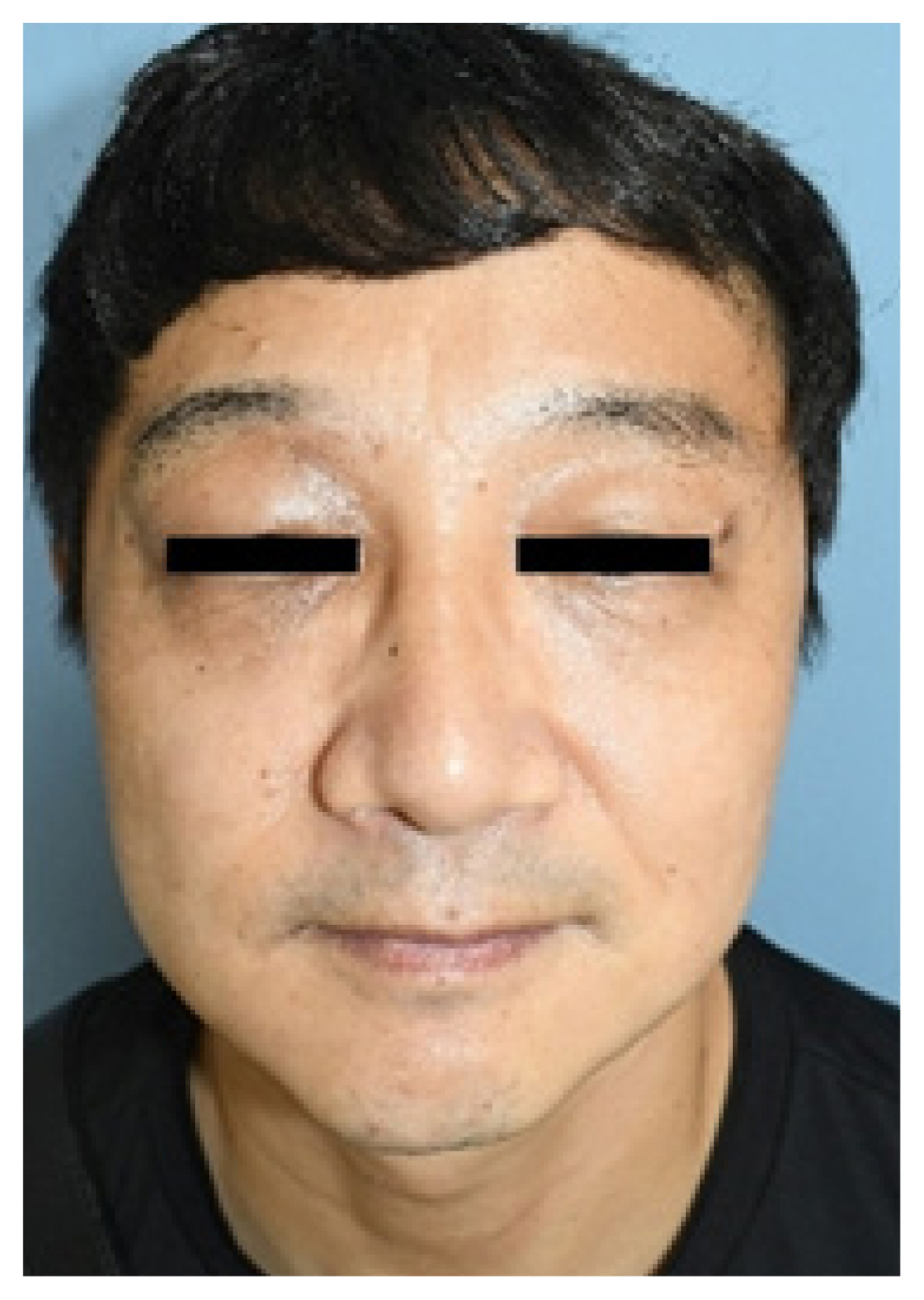
A 43-year-old man visited our clinic with a right upper eyelid mass that had gradually increased in size for 10 years. A physical exam revealed slight ptosis of the right eye and a palpable, firm mass measuring 2×2 cm on his right upper eyelid with no tenderness (Fig. 1). He had no visual disturbances, and the ocular examination was within the normal limits. The past medical history was not specific.
Color Doppler ultrasonography showed heteroechogenicity, well-defined margins, and blood-filled spaces (Fig. 2). Enhanced computed tomography demonstrated a 1.5×1.35×1 cm, circumscribed, heterogeneous, and diffusely enhanced right upper eyelid mass (Fig. 3).
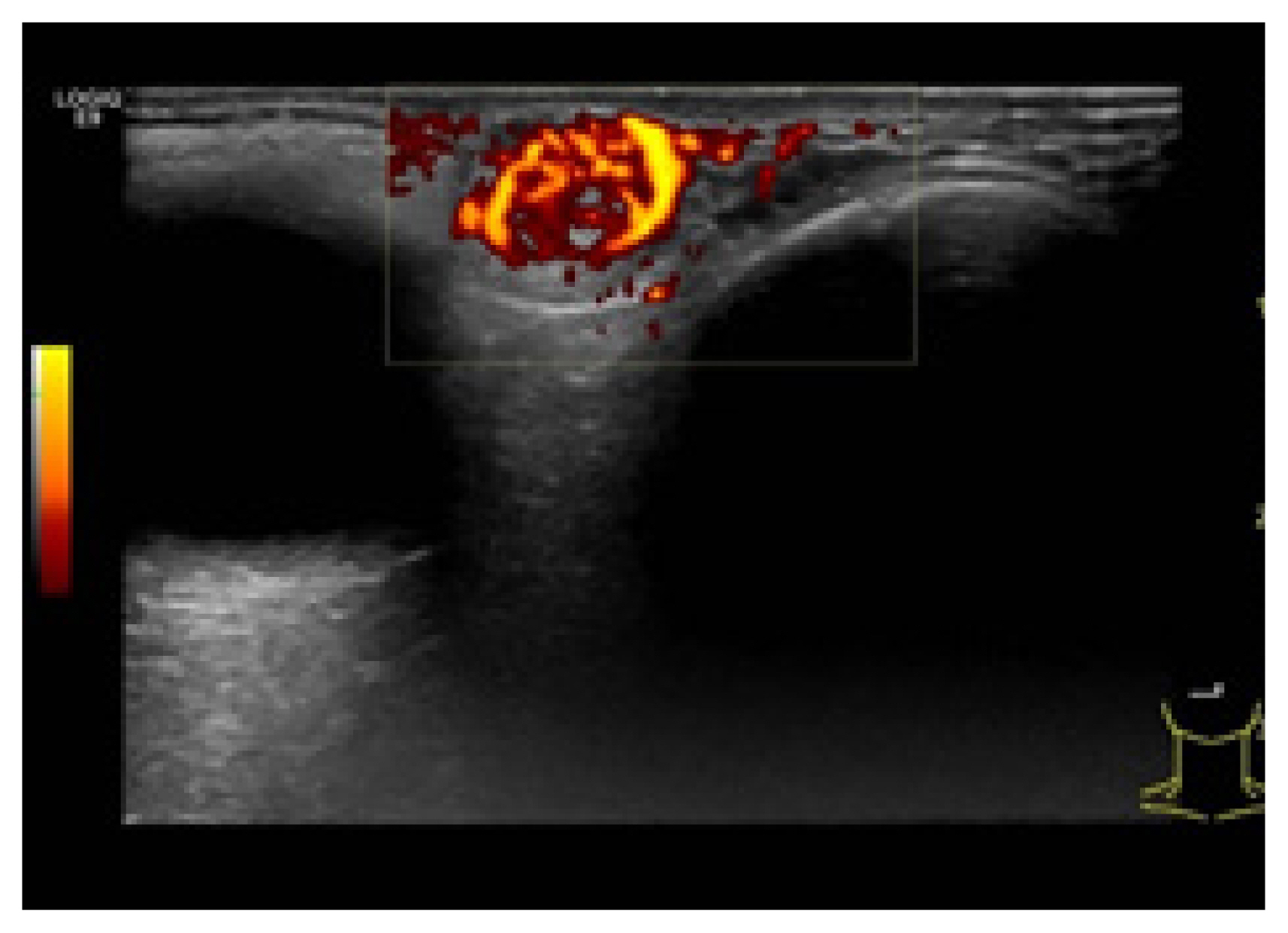
Color Doppler ultrasonography shows heteroechogenicity, awell-defined margin, and blood-filled spaces.
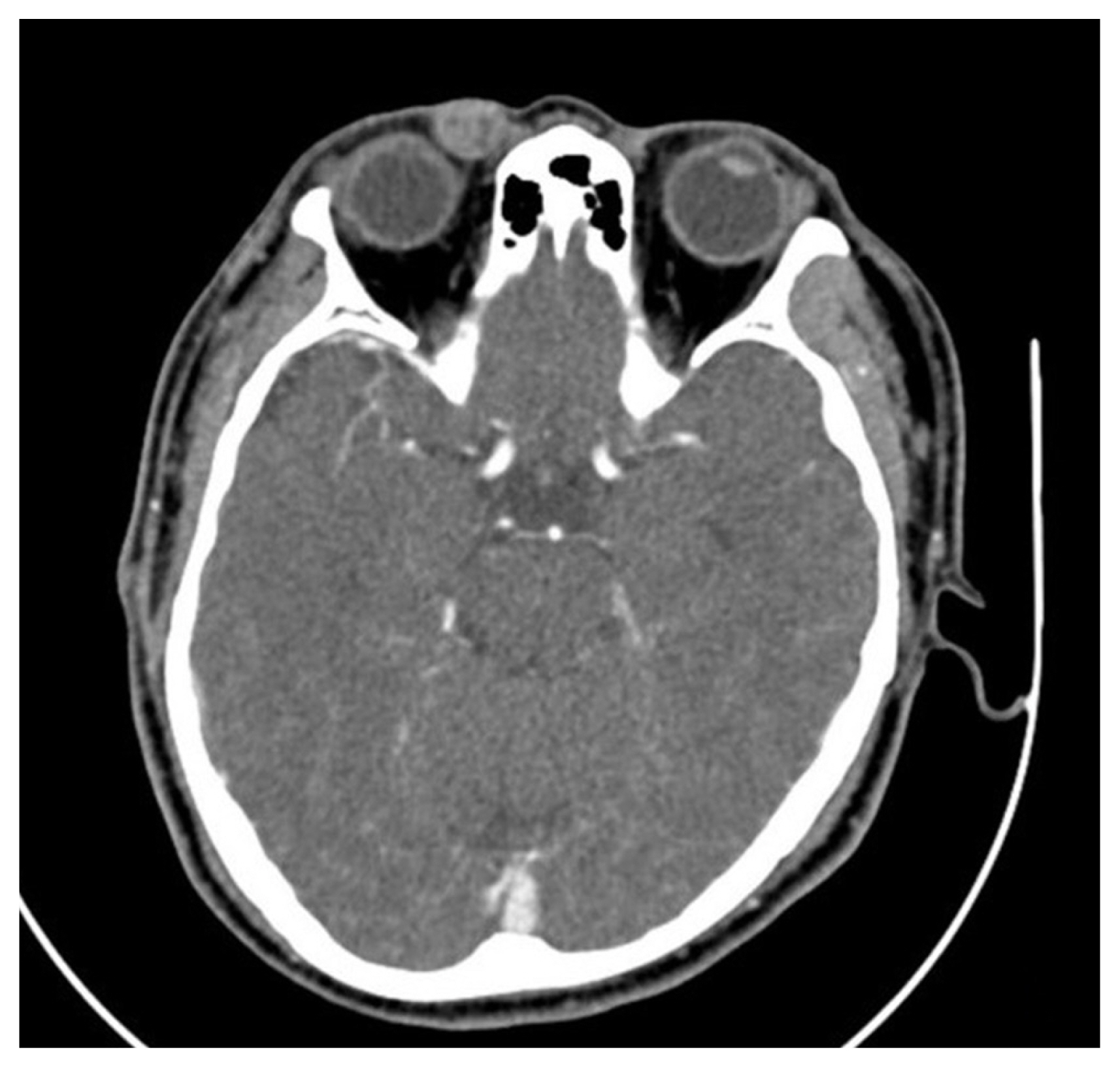
Enhanced computed tomographic scan show a circumscribed soft tissue mass on the right upper eyelid with heterogeneous density.
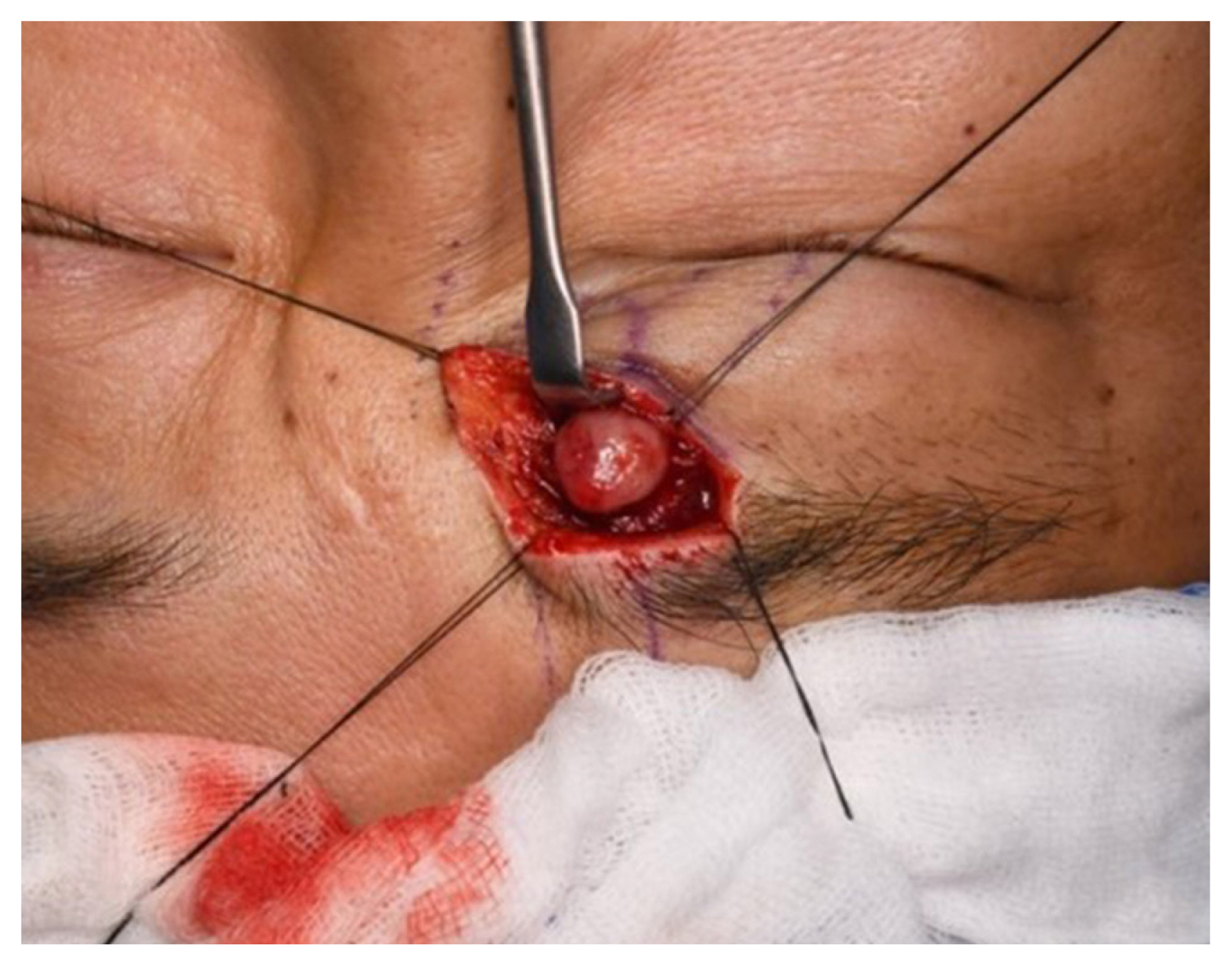
Surgical removal of the tumor was performed with meticulous dissection via the sub-brow approach. The skin and orbicularis oculi muscle were dissected, and a yellow and flesh-colored, 1×1×1 cm, well-encapsulated, round mass was removed (Fig. 4).
Histologically, hematoxylin and eosin staining showed that the upper eyelid mass had a triphasic composition with mature adipose tissue, thick-walled blood vessels and a myoid component with eosinophilic cytoplasm (Fig. 5). Immunohistochemically, the tumor cells were positive for smooth muscle actin (Fig. 6A) and negative for HMB-45 expression (Fig. 6B).

A triphasic composition is seen, with mature adipose tissue, thick-walled blood vessels (circle), and a myoid component (asterisks). Hematoxylin and eosin staining (×10).
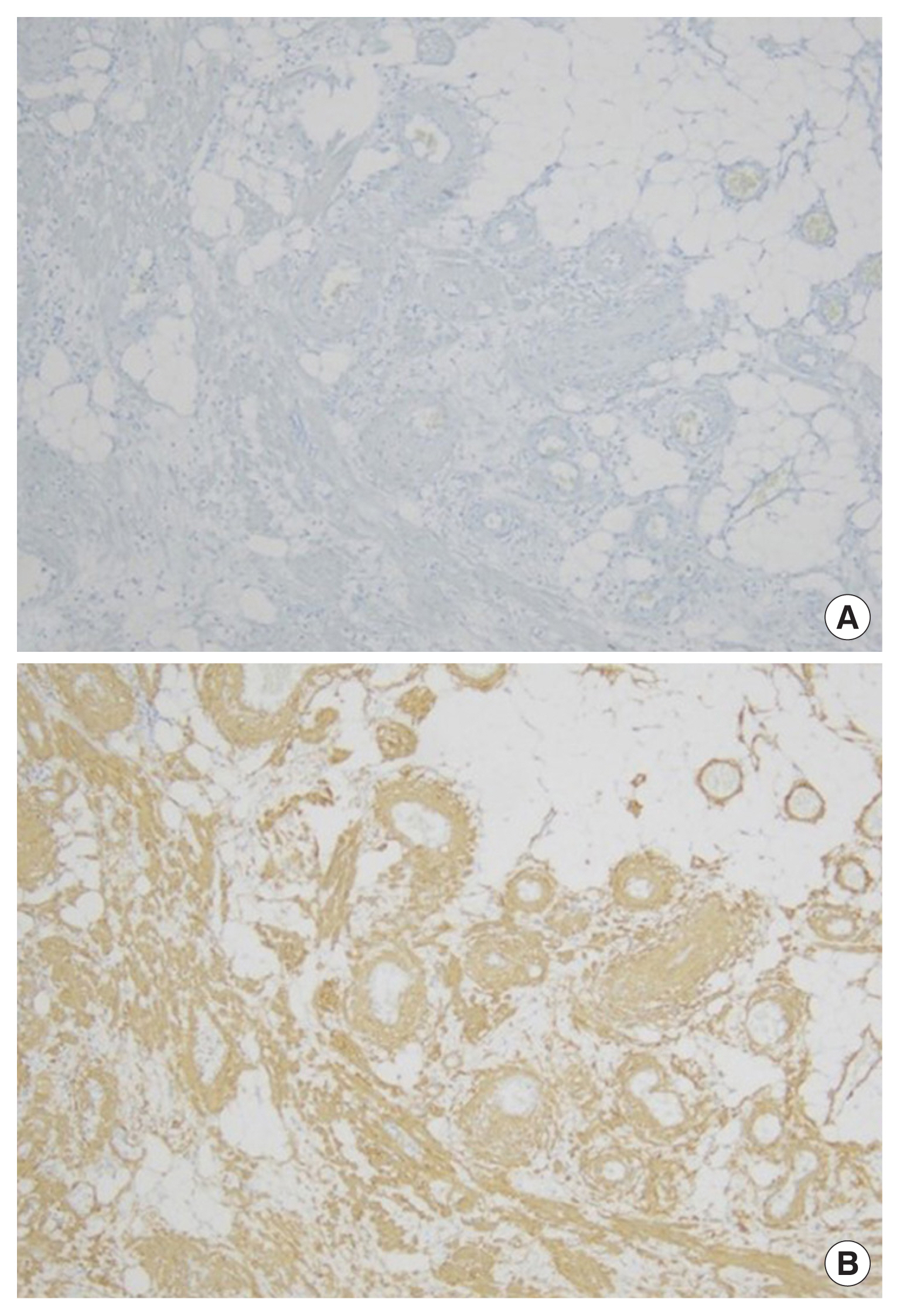
Pathological findings. (A) The smooth muscle component and vascular wall are immunoreactive for smooth muscle actin (smooth muscle actin staining, ×10). (B) No positivity of human melanoma black-45 staining is shown (×10).
These findings confirmed the diagnosis of AML. During regular outpatient follow-up, the patient reported no discomfort on the upper eyelid, and no recurrence was noted at 6 months of follow-up (Fig. 7).
DISCUSSION
AML is known to be a benign tumor that usually occurs in the kidney. AML is a hamartomatous lesion, rather than neoplastic. A hamartoma is a focal malformation in that it is composed of normal tissue elements but grows in a disorderly fashion [6]. It also belongs to a family of tumors arising from PEComas. PEComas have histopathological features of epithelioid cells along a perivascular distribution and characteristic immunohistochemistry with the co-expression of myoid and melanocytic markers (HMB-45).
Most AMLs are benign, but a malignant form also has been reported [7]. The malignant variant of AML is typically the epithelioid subtype, and it has been documented in both the kidney and liver [8]. Furthermore, AMLs may occur sporadically or in association with tuberous sclerosis complex [8].
The overall incidence of AMLs is estimated to be 0.3% in the general population [9]. Extrarenal AML is extremely rare, accounting for only 0.1% to 0.3% of cases of AML. Extrarenal AMLs have been reported in the liver, medi¬astinum, heart, spinal cord, spermatic cord, penis, lung, vaginal wall, Fallopian tube, oral cavity, pharynx, nasal cavity, and skin [5].
Cutaneous AMLs are unique in that these tumors lack reactivity for the melanin-associated antigen HMB-45, which is similar to some AMLs from the skin, head, and neck. Because of the small number of reported cases, further investigation is needed to establish whether these HMB-45-negative AMLs are a new variant of PEComas. Only seven cases of ocular PEComas have been reported; two cases were negative for melanocytic markers, and five cases were positive [10].
Our report presents a single case, but it is meaningful as a rare case of HMB-45-negative AML occurring in the upper eyelid. More broadly, there are various lumps that occur on the face. Some examples of these masses with relatively high prevalence include epidermal cysts, pilomatricomas, osteomas, lipomas, dermoid cysts, and cancerous regions [11,12].
Based on our case, we present some considerations to help clinicians identify AML in the facial area, which is rare but possible. Being aware of this possibility may prompt the operator to perform an imaging evaluation such as color Doppler ultrasonography or computed tomography before surgery, and then to perform a particularly effective and meticulous removal of the mass with consideration of the bleeding tendency of lumps in which thick-walled blood vessels exist, such as AMLs.
In addition, since some previous cases have described a malignant tendency in retroperitoneal AML, despite an initial benign appearance, a histological differential diagnosis is essential.
Extrarenal AMLs have rarely been reported as malignant, but in light of their increasingly widespread description in case reports, it is strongly recommended to make a pathological diagnosis, fully explain to the patient that AML can develop into a malignancy if atypical cells are seen, and recommend regular follow-up.
Abbreviations
AML
angiomyolipoma
HMB-45
human melanoma black-45
PEComa
perivascular epithelioid cell tumor
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Ethical approval
The report was approved by the Institutional Review Board of Chosun University Hospital (IRB No. 2022-11-005).
Patient consent
The patient provided written informed consent for the publication use of his images.
Author contributions
Conceptualization: Young Jun Kim, Min Hyub Choi. Formal analysis: Young Jun Kim, Woo Young Choi. Writing-original draft: Young Jun Kim. Writing-review & editing: Woo Young Choi. Investigation: Young Jun Kim. Resources: Young Jun Kim, Ji Seon Cheon, Woo Young Choi.
Funding
None.