1. Latham K, Buchanan EP, Suver D, Gruss JS. Neurofibromatosis of the head and neck: classification and surgical management. Plast Reconstr Surg 2015;135:845-55.


2. Huson SM, Harper PS, Compston DA. Von Recklinghausen neurofibromatosis: a clinical and population study in southeast Wales. Brain 1988;111(Pt 6):1355-81.


3. Neurofibromatosis. Natl Inst Health Consens Dev Conf Consens Statement 1987;6:1-7.
5. Cimino PJ, Gutmann DH. Neurofibromatosis type 1. Handb Clin Neurol 2018;148:799-811.


6. Packer RJ, Fisher MJ, Cutter G, Cole-Plourde K, Korf BR. Neurofibromatosis clinical trial consortium. J Child Neurol 2018;33:82-91.


7. Bettegowda C, Upadhayaya M, Evans DG, Kim A, Mathios D, Hanemann CO, et al. Genotype-phenotype correlations in neurofibromatosis and their potential clinical use. Neurology 2021;97(7 Suppl 1):S91-8.


8. Rauen KA, Huson SM, Burkitt-Wright E, Evans DG, Farschtschi S, Ferner RE, et al. Recent developments in neurofibromatoses and RASopathies: management, diagnosis and current and future therapeutic avenues. Am J Med Genet A 2015;167A:1-10.


9. Evans DG, Howard E, Giblin C, Clancy T, Spencer H, Huson SM, et al. Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A 2010;152A:327-32.


10. Friedman JM. Epidemiology of neurofibromatosis type 1. Am J Med Genet 1999;89:1-6.


12. Uusitalo E, Rantanen M, Kallionpaa RA, Poyhonen M, Leppavirta J, Yla-Outinen H, et al. Distinctive cancer associations in patients with neurofibromatosis type 1. J Clin Oncol 2016;34:1978-86.


13. Ly KI, Blakeley JO. The diagnosis and management of neurofibromatosis type 1. Med Clin North Am 2019;103:1035-54.


14. Skuse GR, Kosciolek BA, Rowley PT. Molecular genetic analysis of tumors in von Recklinghausen neurofibromatosis: loss of heterozygosity for chromosome 17. Genes Chromosomes Cancer 1989;1:36-41.


15. Messiaen LM, Callens T, Mortier G, Beysen D, Vandenbroucke I, van Roy N, et al. Exhaustive mutation analysis of the NF1 gene allows identification of 95% of mutations and reveals a high frequency of unusual splicing defects. Hum Mutat 2000;15:541-55.


16. Weiss B, Bollag G, Shannon K. Hyperactive Ras as a therapeutic target in neurofibromatosis type 1. Am J Med Genet 1999;89:14-22.


17. Daston MM, Scrable H, Nordlund M, Sturbaum AK, Nissen LM, Ratner N. The protein product of the neurofibromatosis type 1 gene is expressed at highest abundance in neurons, Schwann cells, and oligodendrocytes. Neuron 1992;8:415-28.


18. Ruggieri M, Huson SM. The clinical and diagnostic implications of mosaicism in the neurofibromatoses. Neurology 2001;56:1433-43.


19. Upadhyaya M, Huson SM, Davies M, Thomas N, Chuzhanova N, Giovannini S, et al. An absence of cutaneous neurofibromas associated with a 3-bp inframe deletion in exon 17 of the NF1 gene (c.2970-2972 delAAT): evidence of a clinically significant NF1 genotype-phenotype correlation. Am J Hum Genet 2007;80:140-51.


20. Pinna V, Lanari V, Daniele P, Consoli F, Agolini E, Margiotti K, et al. p.Arg1809Cys substitution in neurofibromin is associated with a distinctive NF1 phenotype without neurofibromas. Eur J Hum Genet 2015;23:1068-71.


22. Koczkowska M, Chen Y, Callens T, Gomes A, Sharp A, Johnson S, et al. Genotype-phenotype correlation in NF1: evidence for a more severe phenotype associated with missense mutations affecting NF1 codons 844-848. Am J Hum Genet 2018;102:69-87.


24. DeBella K, Szudek J, Friedman JM. Use of the national institutes of health criteria for diagnosis of neurofibromatosis 1 in children. Pediatrics 2000;105(3 Pt 1):608-14.


25. Hirsch NP, Murphy A, Radcliffe JJ. Neurofibromatosis: clinical presentations and anaesthetic implications. Br J Anaesth 2001;86:555-64.


28. Wu M, Wallace MR, Muir D. Tumorigenic properties of neurofibromin-deficient Schwann cells in culture and as syngrafts in Nf1 knockout mice. J Neurosci Res 2005;82:357-67.


29. Rutkowski JL, Wu K, Gutmann DH, Boyer PJ, Legius E. Genetic and cellular defects contributing to benign tumor formation in neurofibromatosis type 1. Hum Mol Genet 2000;9:1059-66.


31. Munchhof AM, Li F, White HA, Mead LE, Krier TR, Fenoglio A, et al. Neurofibroma-associated growth factors activate a distinct signaling network to alter the function of neurofibromindeficient endothelial cells. Hum Mol Genet 2006;15:1858-69.


34. Williams VC, Lucas J, Babcock MA, Gutmann DH, Korf B, Maria BL. Neurofibromatosis type 1 revisited. Pediatrics 2009;123:124-33.


36. Roth TM, Petty EM, Barald KF. The role of steroid hormones in the NF1 phenotype: focus on pregnancy. Am J Med Genet A 2008;146A:1624-33.


37. Riccardi VM. An overview of NF-1: dysplasia and neoplasia. In: Friedman JM, Gutmann DH, MacCollin M, Riccardi VM, editors. Neurofibromatosis: phenotype, natural history, and pathogenesis. Baltimore: The Johns Hopkins University Press; 1992. p. 28-33.
38. Brenaut E, Nizery-Guermeur C, Audebert-Bellanger S, Ferkal S, Wolkenstein P, Misery L, et al. Clinical characteristics of pruritus in neurofibromatosis 1. Acta Derm Venereol 2016;96:398-9.


39. Aponte-Lopez A, Munoz-Cruz S. Mast cells in the tumor microenvironment. Adv Exp Med Biol 2020;1273:159-73.


40. Kamide R, Nomura N, Niimura M. Characterization of mast cells residing in cutaneous neurofibromas. Dermatologica 1989;179 Suppl 1:124.


41. Riccardi VM. Cutaneous manifestation of neurofibromatosis: cellular interaction, pigmentation, and mast cells. Birth Defects Orig Artic Ser 1981;17:129-45.
42. Ortonne N, Wolkenstein P, Blakeley JO, Korf B, Plotkin SR, Riccardi VM, et al. Cutaneous neurofibromas: current clinical and pathologic issues. Neurology 2018;91(2 Suppl 1):S5-13.


43. Beert E, Brems H, Daniels B, De Wever I, Van Calenbergh F, Schoenaers J, et al. Atypical neurofibromas in neurofibromatosis type 1 are premalignant tumors. Genes Chromosomes Cancer 2011;50:1021-32.


44. Wolkenstein P, Zeller J, Revuz J, Ecosse E, Leplege A. Quality-of-life impairment in neurofibromatosis type 1: a cross-sectional study of 128 cases. Arch Dermatol 2001;137:1421-5.


45. Verma SK, Riccardi VM, Plotkin SR, Weinberg H, Anderson RR, Blakeley JO, et al. Considerations for development of therapies for cutaneous neurofibroma. Neurology 2018;91(2 Suppl 1):S21-30.


49. Korf BR. Plexiform neurofibromas. Am J Med Genet 1999;89:31-7.


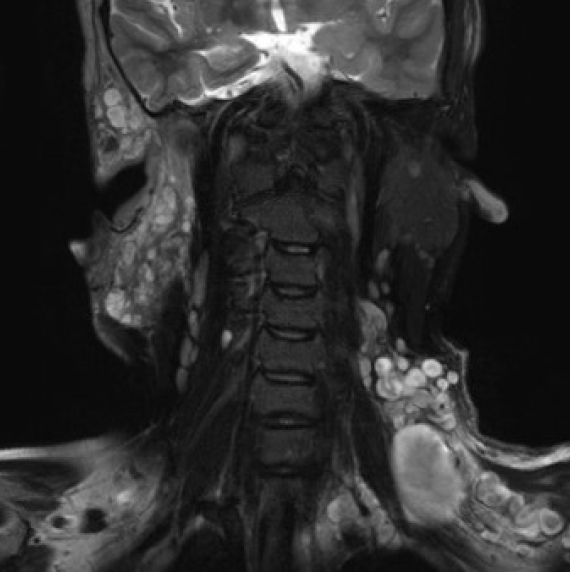
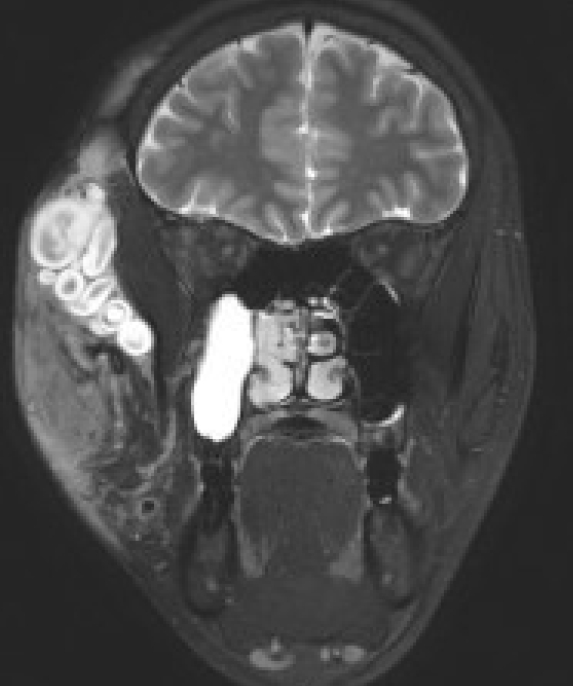
50. Mautner VF, Hartmann M, Kluwe L, Friedrich RE, Funsterer C. MRI growth patterns of plexiform neurofibromas in patients with neurofibromatosis type 1. Neuroradiology 2006;48:160-5.


51. Korf BR. Malignancy in neurofibromatosis type 1. Oncologist 2000;5:477-85.


53. Tucker T, Wolkenstein P, Revuz J, Zeller J, Friedman JM. Association between benign and malignant peripheral nerve sheath tumors in NF1. Neurology 2005;65:205-11.


54. Dombi E, Solomon J, Gillespie AJ, Fox E, Balis FM, Patronas N, et al. NF1 plexiform neurofibroma growth rate by volumetric MRI: relationship to age and body weight. Neurology 2007;68:643-7.


55. Miller DT, Freedenberg D, Schorry E, Ullrich NJ, Viskochil D, Korf BR, et al. Health supervision for children with neurofibromatosis type 1. Pediatrics 2019;143:e20190660.


63. Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol 2014;13:834-43.


64. Evans DG, Salvador H, Chang VY, Erez A, Voss SD, Schneider KW, et al. Cancer and central nervous system tumor surveillance in pediatric neurofibromatosis 1. Clin Cancer Res 2017;23:e46-53.


65. Carli M, Ferrari A, Mattke A, Zanetti I, Casanova M, Bisogno G, et al. Pediatric malignant peripheral nerve sheath tumor: the Italian and German soft tissue sarcoma cooperative group. J Clin Oncol 2005;23:8422-30.


66. Nguyen R, Jett K, Harris GJ, Cai W, Friedman JM, Mautner VF. Benign whole body tumor volume is a risk factor for malignant peripheral nerve sheath tumors in neurofibromatosis type 1. J Neurooncol 2014;116:307-13.


67. King AA, Debaun MR, Riccardi VM, Gutmann DH. Malignant peripheral nerve sheath tumors in neurofibromatosis 1. Am J Med Genet 2000;93:388-92.


69. Malbari F, Spira M, B Knight P, Zhu C, Roth M, Gill J, et al. Malignant peripheral nerve sheath tumors in neurofibromatosis: impact of family history. J Pediatr Hematol Oncol 2018;40:e359-63.


71. Woodruff JM. Pathology of tumors of the peripheral nerve sheath in type 1 neurofibromatosis. Am J Med Genet 1999;89:23-30.


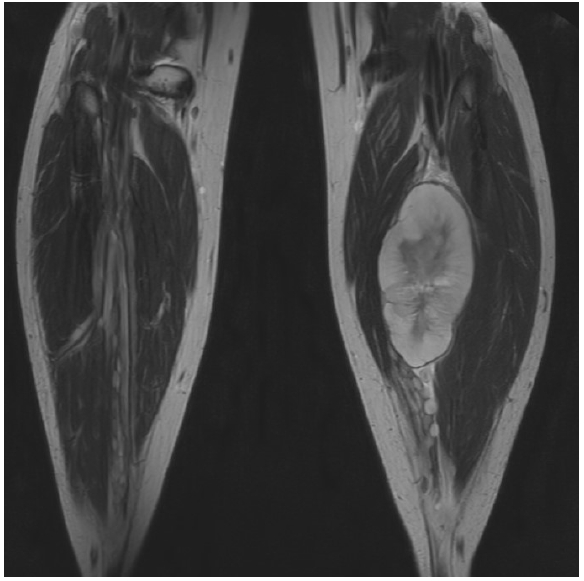
72. Wasa J, Nishida Y, Tsukushi S, Shido Y, Sugiura H, Nakashima H, et al. MRI features in the differentiation of malignant peripheral nerve sheath tumors and neurofibromas. AJR Am J Roentgenol 2010;194:1568-74.


74. Ferner RE, Golding JF, Smith M, Calonje E, Jan W, Sanjayanathan V, et al. [18F]2-fluoro-2-deoxy-D-glucose positron emission tomography (FDG PET) as a diagnostic tool for neurofibromatosis 1 (NF1) associated malignant peripheral nerve sheath tumours (MPNSTs): a long-term clinical study. Ann Oncol 2008;19:390-4.


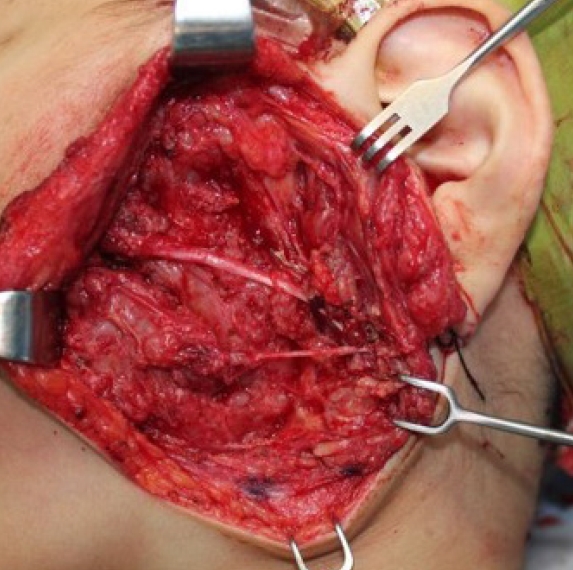
75. Martin E, Pendleton C, Verhoef C, Spinner RJ, Coert JH, MONACO collaborators. Morbidity and function loss after resection of malignant peripheral nerve sheath tumors. Neurosurgery 2022;90:354-64.


76. Dunn GP, Spiliopoulos K, Plotkin SR, Hornicek FJ, Harmon DC, Delaney TF, et al. Role of resection of malignant peripheral nerve sheath tumors in patients with neurofibromatosis type 1. J Neurosurg 2013;118:142-8.


77. Lu VM, Wang S, Daniels DJ, Spinner RJ, Levi AD, Niazi TN. The clinical course and role of surgery in pediatric malignant peripheral nerve sheath tumors: a database study. J Neurosurg Pediatr 2021;29:92-9.


79. Frustaci S, Gherlinzoni F, De Paoli A, Bonetti M, Azzarelli A, Comandone A, et al. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: results of the Italian randomized cooperative trial. J Clin Oncol 2001;19:1238-47.


80. Sarcoma Meta-analysis Collaboration (SMAC). Adjuvant chemotherapy for localised resectable soft tissue sarcoma in adults. Cochrane Database Syst Rev 2000;(4):CD001419.

81. Ferner RE, Gutmann DH. International consensus statement on malignant peripheral nerve sheath tumors in neurofibromatosis. Cancer Res 2002;62:1573-7.

82. Kroep JR, Ouali M, Gelderblom H, Le Cesne A, Dekker TJ, Van Glabbeke M, et al. First-line chemotherapy for malignant peripheral nerve sheath tumor (MPNST) versus other histological soft tissue sarcoma subtypes and as a prognostic factor for MPNST: an EORTC soft tissue and bone sarcoma group study. Ann Oncol 2011;22:207-14.


83. Friedman JM, Birch PH. Type 1 neurofibromatosis: a descriptive analysis of the disorder in 1,728 patients. Am J Med Genet 1997;70:138-43.


84. Jackson IT, Carbonnel A, Potparic Z, Shaw K. Orbitotemporal neurofibromatosis: classification and treatment. Plast Reconstr Surg 1993;92:1-11.


85. Arrington DK, Danehy AR, Peleggi A, Proctor MR, Irons MB, Ullrich NJ. Calvarial defects and skeletal dysplasia in patients with neurofibromatosis type 1. J Neurosurg Pediatr 2013;11:410-6.


87. Lee L, Yan YH, Pharoah MJ. Radiographic features of the mandible in neurofibromatosis: a report of 10 cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996;81:361-7.


89. Riccardi VM. A controlled multiphase trial of ketotifen to minimize neurofibroma-associated pain and itching. Arch Dermatol 1993;129:577-81.


90. Gupta A, Cohen BH, Ruggieri P, Packer RJ, Phillips PC. Phase I study of thalidomide for the treatment of plexiform neurofibroma in neurofibromatosis 1. Neurology 2003;60:130-2.


92. Jakacki RI, Dombi E, Steinberg SM, Goldman S, Kieran MW. Ullrich NJ, et al. Phase II trial of pegylated interferon alfa-2b in young patients with neurofibromatosis type 1 and unresectable plexiform neurofibromas. Neuro Oncol 2017;19:289-97.

93. Cichowski K, Jacks T. NF1 tumor suppressor gene function: narrowing the GAP. Cell 2001;104:593-604.


96. Gross AM, Dombi E, Widemann BC. Current status of MEK inhibitors in the treatment of plexiform neurofibromas. Childs Nerv Syst 2020;36:2443-52.