Efficacy and safety of equine cartilage for rhinoplasty: a multicenter double-blind non-inferiority randomized confirmatory clinical trial
Article information
Abstract
Background
The efficacy and safety of equine cartilage as a competent xenograft material for rhinoplasty were evaluated and compared to the outcomes of rhinoplasty using silicone implants.
Methods
We performed a multicenter, double-blind, non-inferiority, and randomized confirmatory study. Fifty-six patients were randomized 1:1 to the study group (using MegaCartilage-E) and control group (using silicone implants). The Rhinoplasty Outcome Evaluation (ROE) score, photo documentation, Global Aesthetic Improvement Scale (GAIS), and adverse event data were obtained until 12 months after surgery. The primary efficacy, which is the change in ROE score 6 months after surgery, was assessed in the modified intention-to-treat set. The secondary efficacy was evaluated in the per-protocol set by assessing the change in ROE score 6 and 12 months after surgery and nasofrontal angle, the height of the nasion, and GAIS 1, 6, and 12 months after surgery.
Results
The change in ROE score of the study group was non-inferior to that of the control group; it increased by 24.26±17.24 in the study group and 18.27±17.60 in the control group (p=0.213). In both groups, all secondary outcome measures increased, but there was no statistical difference. In the safety set, treatment-emergent adverse events occurred in 10 patients (35.71%) in the study group and six patients (21.43%) in the control group (p=0.237). There were 13 adverse device events in the study group and six adverse device events in the control group (p=0.515).
Conclusion
Processed equine cartilage can be used effectively and safely as xenograft material for rhinoplasty.
INTRODUCTION
A substantial amount of dorsal augmentation is frequently needed for Asian rhinoplasty because the nasal bones are flat, and the radix is relatively low in Asians compared to Caucasians [1–3]. Various nasal implants have been used for dorsal augmentation. The ideal nasal implant is thought to be readily available, inexpensive, biocompatible, nontoxic, noncarcinogenic, sterilizable, and easy to sculpt and remove. Furthermore, the ideal implant should maintain its volume and mechanical support over time and resist trauma, infection, and extrusion [4,5]. However, the ideal nasal implant does not exist.
Autologous grafts such as cartilage remain the gold-standard material for nasal implantation [6]. The main advantage is that it has low risk of infection, resorption, rejection, and extrusion [7]. It also elicits minimal inflammatory responses [4]. However, the limitations include limited quantity of available tissue and donor site morbidity [6,8]. It also presents unexpected warping and resorption. To overcome these limitations, homologous costal cartilage has been used because it has “off-the-shelf” accessibility with multiple available sizes. It is also aseptically processed to meet sterile condition and screened to minimize infectious risks [9]. However, the main disadvantage is its limited supply because it needs to be harvested from donated cadavers of young patients as relatively older patients have higher calcium content in their costal cartilages [10]. Alloplastic materials have been widely used for dorsal augmentation because they are relatively affordable. Silicone implants are the most widely used material for augmentation rhinoplasty in Asian countries [11]. Although silicone implants come with the risk of infection, extrusion, and capsular contracture, it is easy to use for significant dorsal augmentation and can be supplied unlimitedly [6].
The research on xenografts is actively progressing because xenografts can overcome the limitation of lack of supply and are affordable compared to the cost of homologous grafts. There have been several studies to test xenografts as potential candidates for nasal implant material for rhinoplasty and nasal reconstruction [12–16]. Herein, the safety and efficacy of equine cartilage as a potential xenograft material for rhinoplasty were evaluated. MegaCartilage-E (L&C Bio, Seoul, Korea) is equine cartilage which is processed by a multi-step washing process and gamma-ray sterilization. To assess its potential as a candidate for rhinoplasty implants, qualitative and quantitative outcomes of rhinoplasty with MegaCartilage-E were assessed and compared with outcomes of rhinoplasty with silicone implants. This study is a multicenter, double-blind (patient-independent investigator), non-inferiority, prospective, and randomized confirmatory clinical trial.
METHODS
Patients and study design
This study was conducted between October 5, 2018, and September 4, 2021, at two tertiary medical centers in Seoul, South Korea. The study protocol was reviewed and approved by the institutional review board of the two independent universities (IRB Nos. 2018-12-001 and 2019-1162).
Sixty-seven patients were screened based on the inclusion and exclusion criteria (Table 1), and 56 patients were enrolled in the study. All the enrolled patients provided written informed consent, and the study was conducted according to the Declaration of Helsinki. Demographic data, medical history, blood and urine test results, and photography were obtained, and physical examinations were performed. The enrolled patients (n=56) were randomly assigned (1:1) to the study and control groups.
Surgical method and implant material
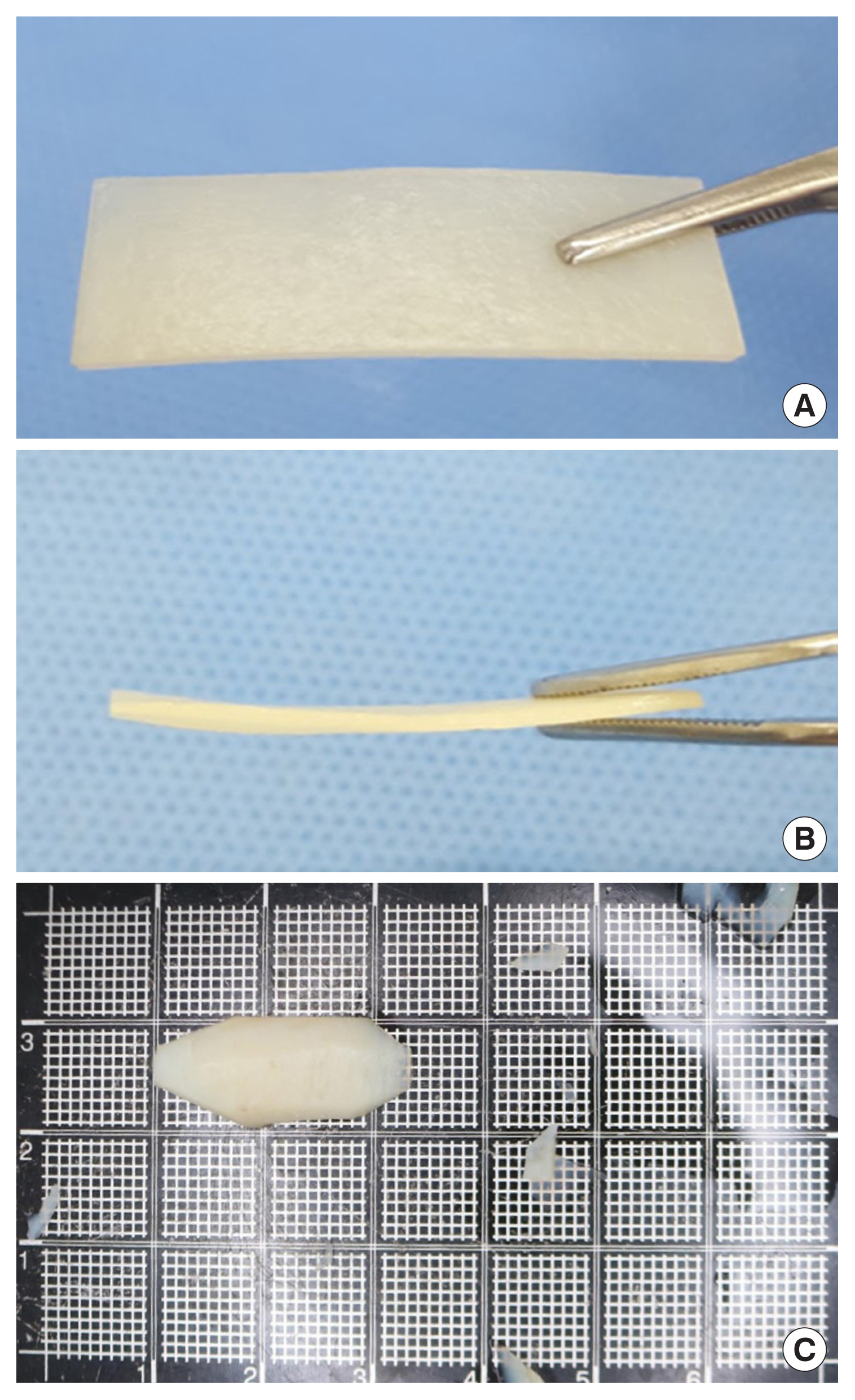
Raw equine cartilage for MegaCartilage-E is harvested from the scapular cartilage of horses (younger than 36-month-old). After the removal of the surrounding tissue, the cartilage was carved into the standardized size [4 cm (length)×0.7–0.9 cm (width)× 0.25±0.49 cm (thickness)]. Then the material was processed with de-lipidation, decellularization, and virus inactivation. Sterilization was performed using gamma irradiation (35 kGy), and the cartilage was immersed in sterile saline solution to be ready for use.
For the study and control groups, rhinoplasty was performed in the same manner under local anesthesia. A 3–5 mm long incision in the nostril approximately 3 mm from the alar edge or a transcollumellar incision was made. Then, the dissection was performed from the upper nostril to the glabella in the subperiosteal plane. Next, the assigned nasal implant was carved and inserted. The incision was closed with 6-0 Vicryl (polyglactin 910) or 7-0 catgut. Skin taping was applied over the dorsum of the nose, and the external nasal splint was maintained for 3 days to prevent malpositioning of the implant.
In the control group, rhinoplasty was performed with the silicone implant (Bistool SOFTXiL, Seoul, Korea), whereas Mega-Cartilage-E (L&C Bio) was used in the study group (Fig. 1). Patients were blinded to the type of implant used for their rhinoplasty even after surgery and the operator to the type of implant that would be used until the operation.
Assessment
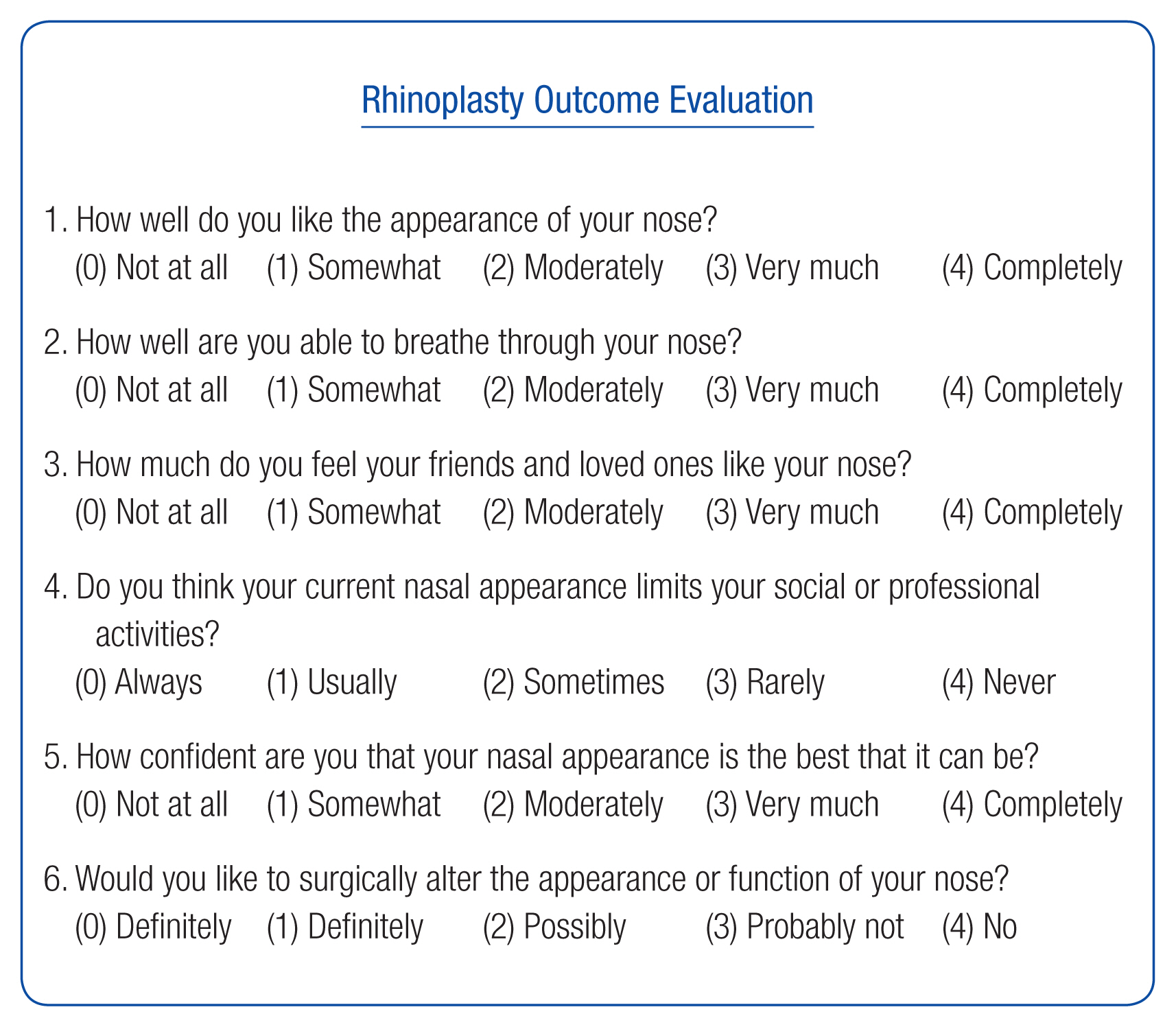
The efficacy was evaluated using the Rhinoplasty Outcome Evaluation (ROE) questionnaire and photo documentation before surgery and 1, 6, and 12 months after surgery. Also, Global Aesthetic Improvement Scale (GAIS) evaluation was evaluated at every visit after surgery. The ROE is a quick and easy-to-perform self-reported questionnaire. It is a standardized and reliable method of evaluating the quality of life following rhinoplasty in terms of physical, mental, and social aspects (Fig. 2) [17]. It is composed of five questions about nasal shape and one about nasal breathing. Each question is scored on a scale of 0–4, where 0 is the most negative answer and 4, the most positive. The sum of all the scores was divided by 24 and multiplied by 100.
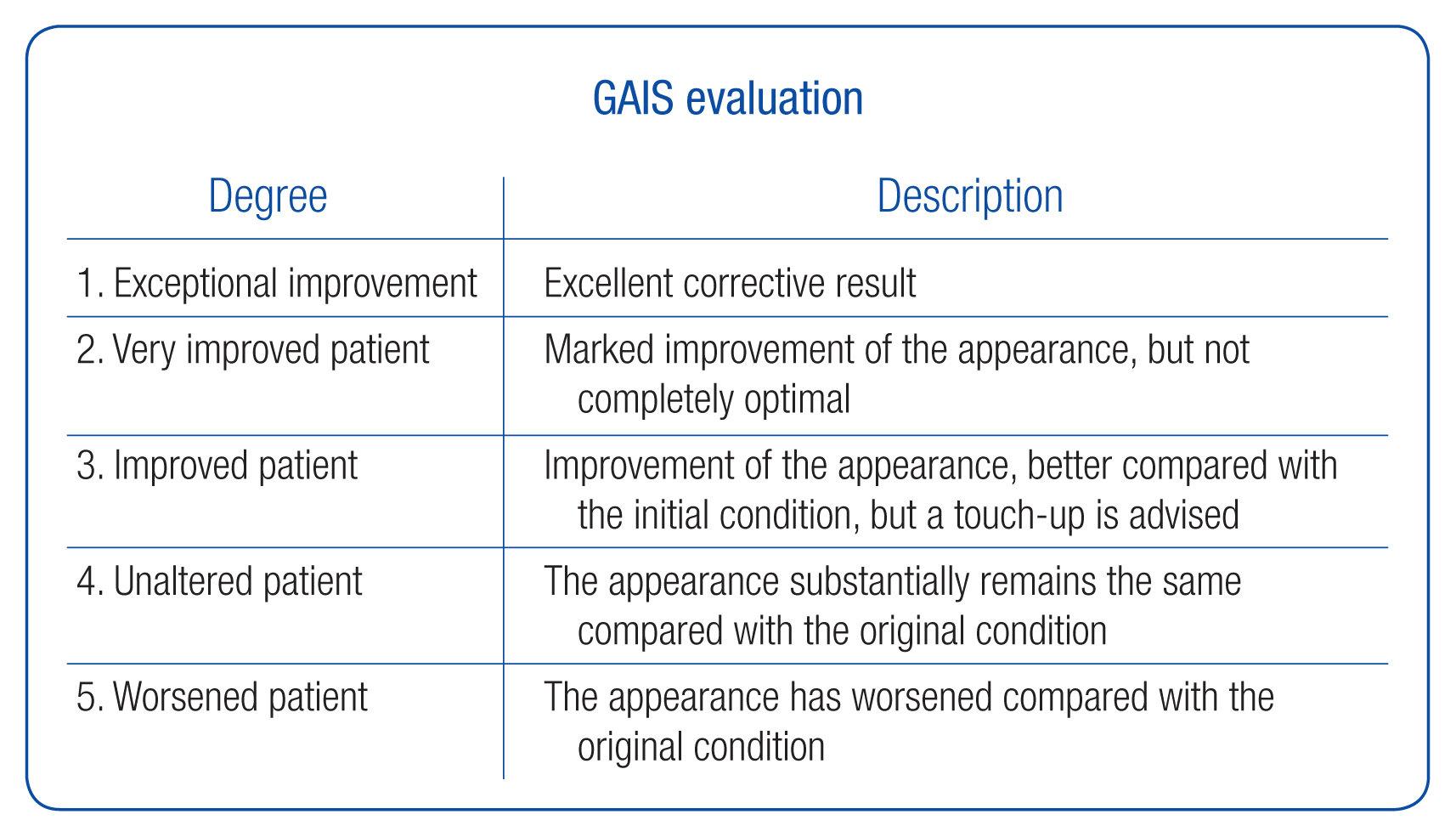
For quantitative measurement, standardized frontal and lateral view photographs were obtained (Fig. 3). The nasofrontal angle and the height of nasion were measured by an independent investigator who was not involved in the surgery (Fig. 4). The investigator was blinded to the type of implant and timing of photography. The investigator’s satisfaction was assessed by GAIS, which is a 5-grade scale (Fig. 5). To assess safety, any adverse events were documented at every visit.
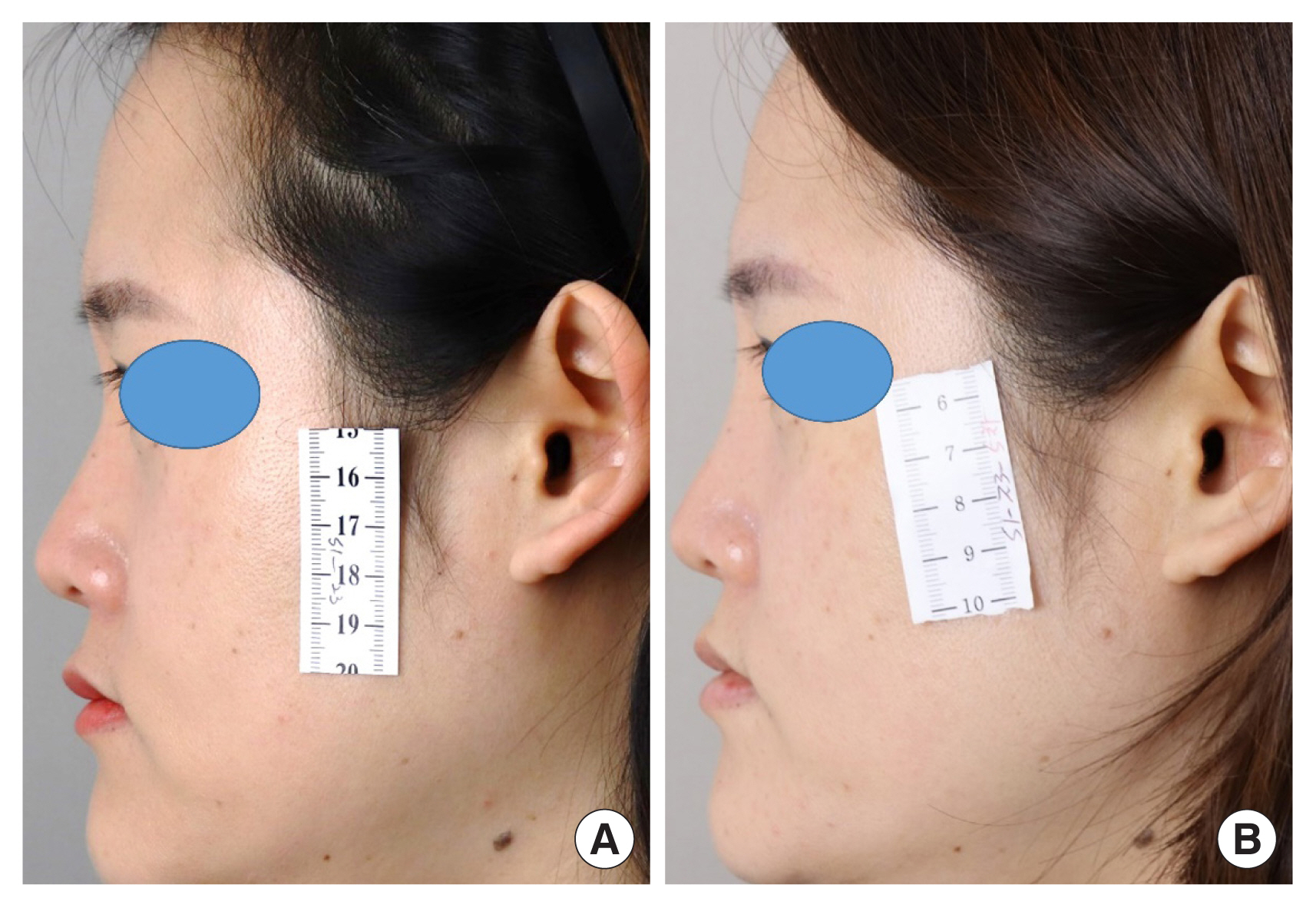
Lateral view photographs of the patient. (A) Preoperative photograph. (B) Postoperative photograph that was taken 48 weeks after surgery.
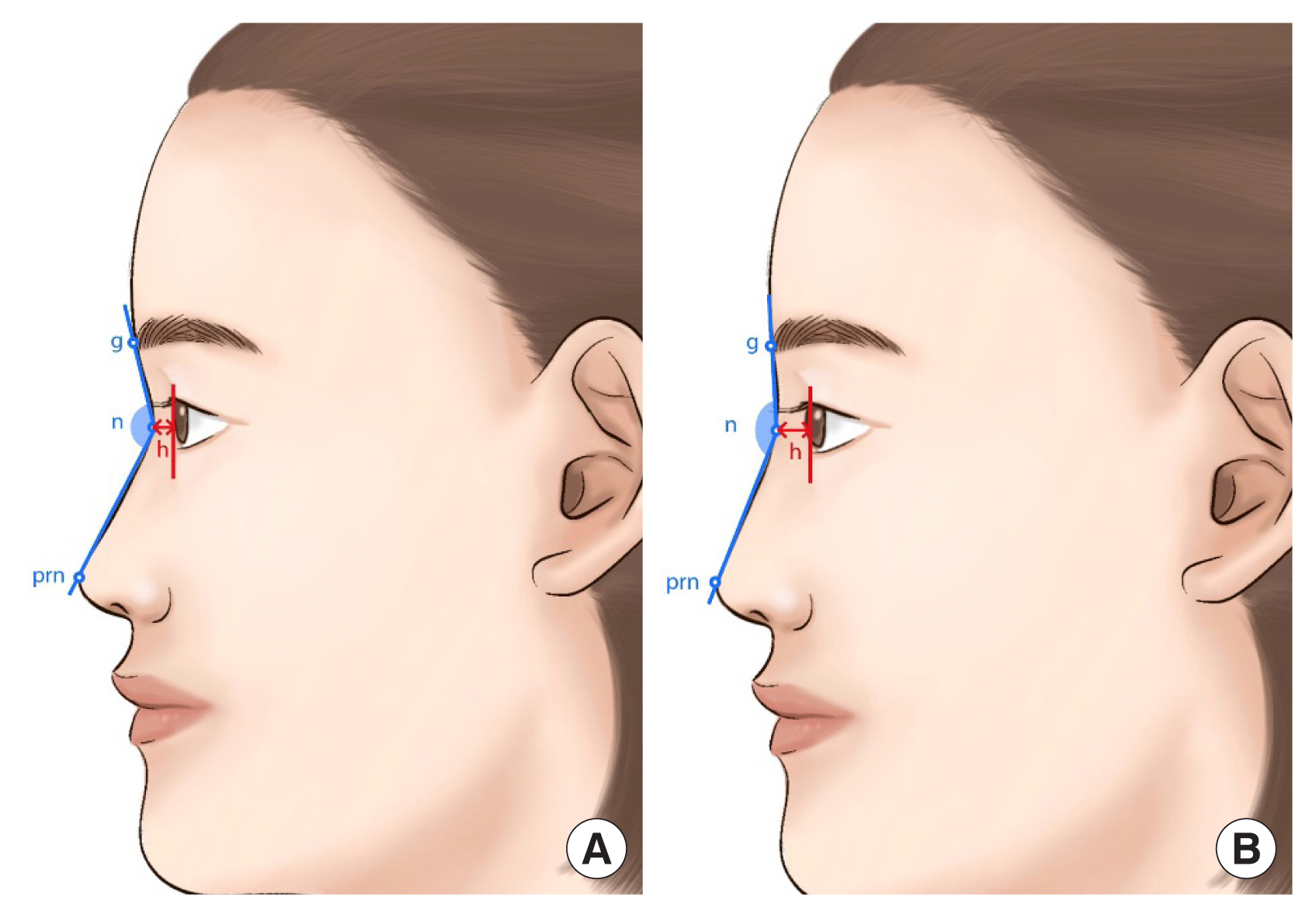
Schematic drawings of patients and measurement methods. The nasofrontal angle was defined as the angle between the line connecting the glabella from the nasion and the line connecting the pronasale from the nasion (blue line). The height of the nasion was defined as the distance from the corneal border to the nasion (red double arrow). (A) Preoperative illustration. (B) Postoperative illustration. The nasofrontal angle and the height of nasion have increased compared to before surgery. g, glabella; n, nasion; prn, pronasale; h, height of the nasion.
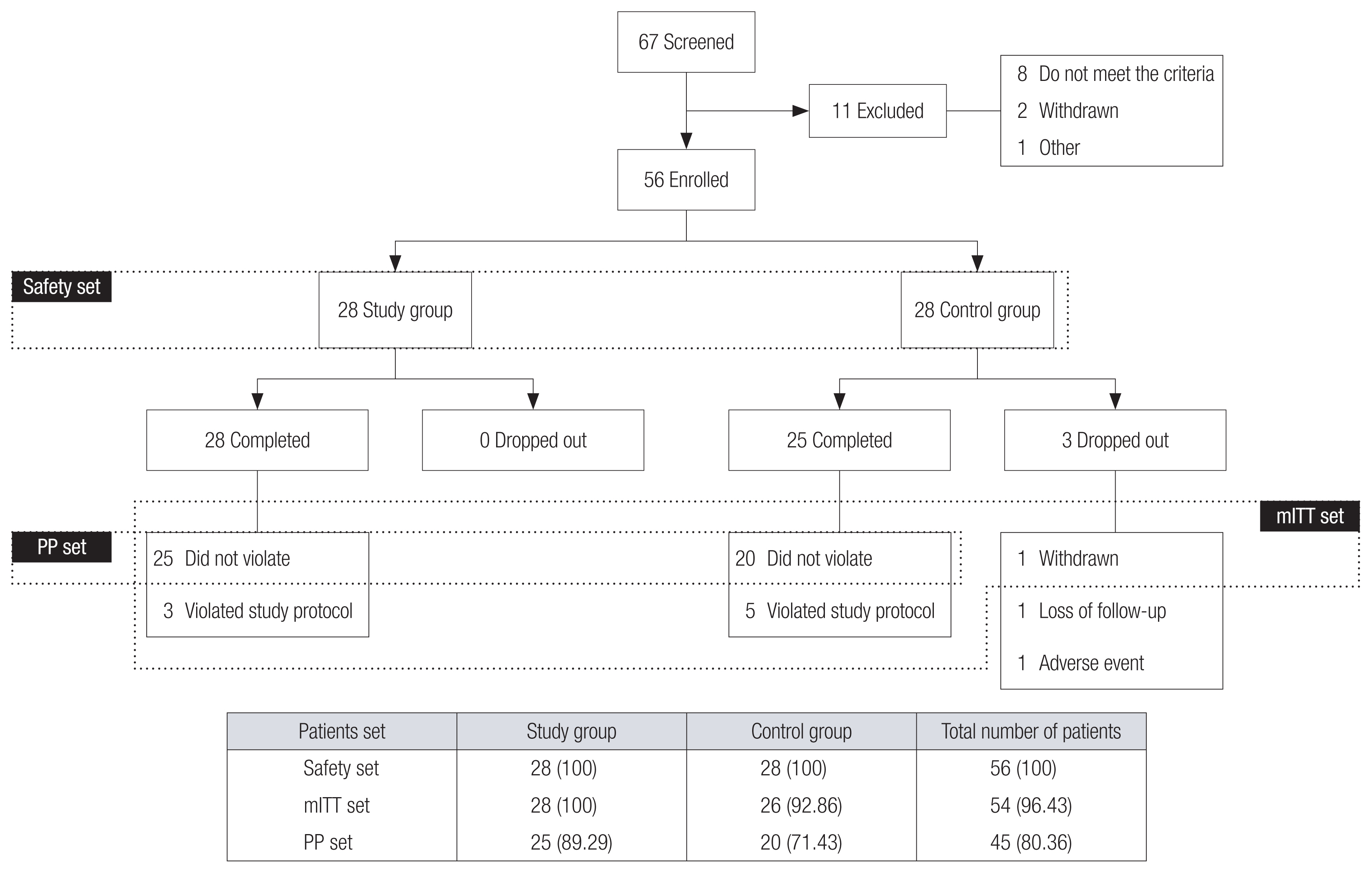
The primary efficacy was assessed by the change in ROE score 6 months after surgery. For the secondary efficacy, the changes in ROE score 1 and 12 months after surgery, nasofrontal angle and height of nasion, and GAIS score of 1, 6, and 12 months after surgery were analyzed. The assessment was performed in three different patient sets (Fig. 6). The modified intention-to-treat (mITT) set was the group of patients that received the primary efficacy assessment. All 28 patients in the study group completed the scheduled study, whereas three patients from the control group dropped out. Of these three patients, two dropped out before the primary efficacy assessment. Thus, all 28 patients from the study group and 26 from the control group were included in the mITT set. The per-protocol (PP) set was defined as the group of patients who completed the entire scheduled study and did not violate any study protocol. The secondary efficacy was assessed in the PP set, which comprised 25 patients from the study group and 20 from the control group. Fifty-six patients who were evaluated for any adverse events more than once were included in the safety set.
Statistical analysis
Data analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). Continuous variables were analyzed using mean, standard deviation (SD), minimum/maximum, or median. For categorical variables, frequency and percentage were proposed. The two-tailed t-test was used to analyze data, and p<0.05 was considered statistically significant.
We computed a sample size of 44 patients, given an alpha error of 2.5% (one-sided independent two-sample t-test) and a power of 80%, with the SD for the primary efficacy being 17.6 (based on a previous study [18]). After considering potential dropouts (20%), the final computed sample size was 56 patients (28 per group). The non-inferiority margin was set at 15 based on clinical data from previous studies [18,19]; that is, the non-inferiority of the study group would be demonstrated if the lower boundary of the one-sided 97.5% confidence interval (CI) for the difference in ROE score between the two groups is greater than −15.
RESULTS
Demographic and laboratory data (mITT set)
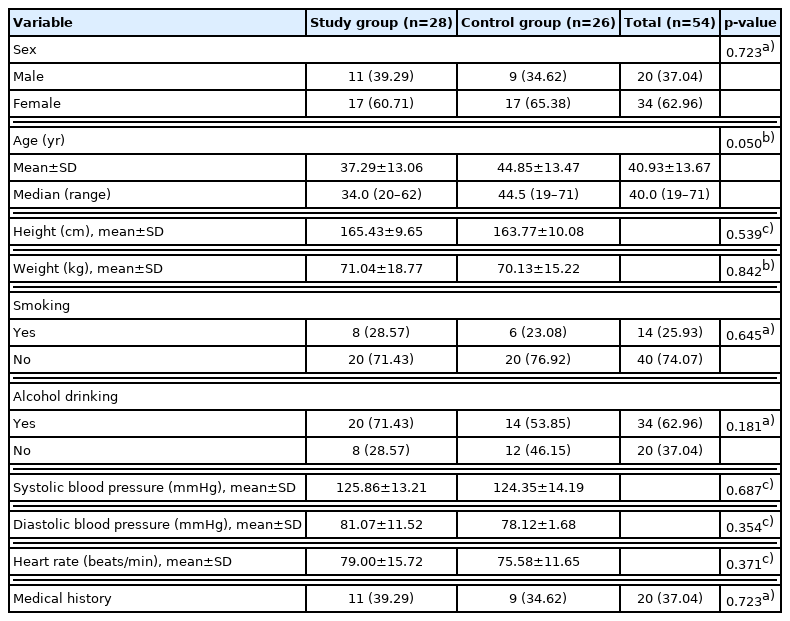
Demographic data were analyzed for the mITT set (Table 2). Of the 28 study group patients, 11 were men (39.29%), and 17 were women (60.17%). In the control group, there were nine men (34.62%) and 17 women (65.38%). There was no statistical difference between the study and control groups (p=0.723). The mean age was 37.29±13.06 in the study group and 44.85± 13.47 in the control group, and there was no statistical difference (p=0.050). Between the study and control groups, weight and height were not statistically different (p=0.539 and p= 0.842, respectively). History of smoking and alcohol consumption, systolic/diastolic blood pressure, heart rate, and the presence of medical history were also not different between the groups (p=0.645, p=0.181, p=0.687, p=0.354, and p=0.723, respectively). In addition, complete blood count, chemistry panel, coagulation test, and urine analysis were also performed, and there was no statistical difference in any specific variables.
Primary efficacy (mITT and PP set)
The primary efficacy was analyzed in the mITT set (Table 3). The mean of ROE score change was 24.26±17.24 in the study group and 18.27±17.60 in the control group. Both changes were statistically significant (p<0.001). The change in ROE score of the study group was non-inferior to that of the control group since the lower boundary of the 97.5% CI was −3.53. According to recommended practice for non-inferiority trials, the non-inferiority was also analyzed in the PP set, and the lower boundary of 97.5% CI was −4.93 (Table 4) [20].
Secondary efficacy (PP set)
As one of the secondary efficacy outcomes, the ROE scores 1 and 12 months after surgery were assessed in the PP set (Table 5). In the study group, the ROE score increased by 26.83±16.05 and 25.00±20.09 at 1 and 12 months after surgery, respectively. In the control group, it increased by 24.17±17.39 and 19.17± 17.95 at 1 and 12 months after surgery, respectively. The change in ROE scores between the two groups was not statistically different at 1 and 12 months after surgery (p=0.596 and p=0.316, respectively).
The nasofrontal angle at 1, 6, and 12 months after surgery and the change from the preoperative status were assessed in the PP set (Table 6). One month after surgery, the nasofrontal angle increased by 10.36°±4.24° in the study group and 7.95°±3.38° in the control group. The change in the nasofrontal angle at 6 months after surgery was 7.92°±4.97° in the study group and 6.73°±3.12° in the control group. Twelve months after surgery, the nasofrontal angle changed by 7.78°±5.06° in the study group and 5.93°±5.06° in the control group. There was no statistical difference between the changes in the two groups at any of these time points (p>0.05) (Fig. 7).
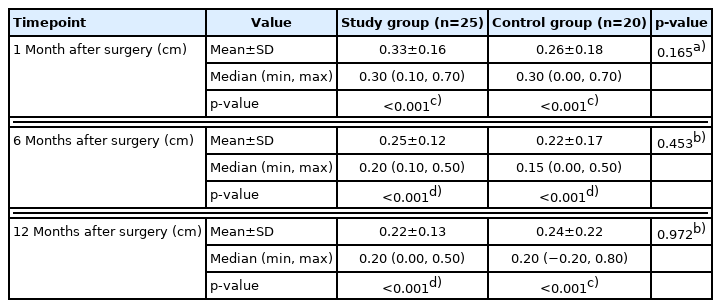
The height of the nasion was also measured, and the change in measurements was compared between the two groups (Table 7). In the study group, the height of the nasion increased by 0.33±0.16 cm, 0.25±0.12 cm, and 0.22±0.13 cm at 1, 6, and 12 months after surgery, respectively. It also increased in the control group by 0.26±0.18 cm, 0.22±0.17 cm, and 0.24±0.22 cm at 1, 6, and 12 months after surgery, respectively. There were no significant differences between the two groups at any of the three-time points (p=0.165, p=0.453, and p=0.972, respectively); however, all the changes had statistical significance (p<0.001) (Fig. 8).
The GAIS was also assessed 1, 6, and 12 months after surgery in both groups and compared (Table 8). In both groups, the outcome of rhinoplasty was assessed as “Exceptionally improved,” “Very improved,” or “Improved.” There was no statistical difference between the two groups at any of the three-time points (p=0.592, p=0.103, and p=0.795, respectively).
Safety (safety set)
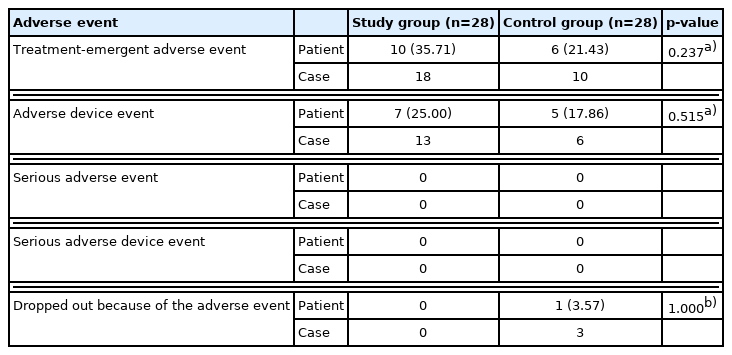
All adverse events were documented and analyzed in the safety set (Table 9). Adverse device effect (ADE) refers to only device-related adverse events, and treatment-emergent adverse event (TEAE) includes all other adverse reactions in the body, including ADE. The number of patients who experienced at least one TEAE was 10 (35.71%) in the study group and six (21.43%) in the control group. Recovery was full for all the 18 TEAEs of the study group (100%) and seven of the control group (70%). Three TEAEs, from which there is ongoing recovery, occurred in a patient, and they were chronic underlying diseases: hypertension, hyperlipidemia, and diabetes mellitus. Thirteen ADEs occurred in seven patients (25.00%) in the study group, and six ADEs occurred in five patients (17.96%) in the control group. There was no statistical difference between the two groups regarding TEAEs and ADEs (p=0.237 and p=0.515). No serious adverse event or serious adverse device event was recorded in either group. Of the 56 patients, only one from the control group dropped out due to adverse events. All ADEs were minor complications, and recovery was complete without sequelae (Table 10).
DISCUSSION
Various implant materials have been used for dorsal augmentation rhinoplasty: autografts (harvested from the same patient), homologous grafts (harvested from cadavers), alloplasts (manufactured from synthetic or semisynthetic materials), and xenografts (derived from another species) [4]. Currently, the first choice for nasal implants is autograft harvested from auricular cartilage, nasal septum, or rib cartilage. The nasal framework is made of hyaline cartilage, which provides more rigid yet elastic support than elastic cartilage [21]. The auricular cartilage is less used for dorsal augmentation not only because of its elastic phenotype but also its natural curvature and warping tendency over time. The septal cartilage is hyaline cartilage and has a straight form [22]. However, significant dorsal augmentation with septal cartilage is often limited by lack of availability. The autologous rib cartilage has relatively abundant availability [23], is easy to carve, and provides good support due to its hyaline phenotype. Thus, it is used as the first choice for dorsal augmentation [24].
However, in addition to warping and progressive calcification, potential morbidity at the donor site is the main disadvantage of autologous rib cartilage. To overcome this, homologous rib cartilage was introduced as a substitute. In a meta-analysis, there was no difference between autologous and homologous rib cartilage grafts in terms of warping, resorption, infection, contour irregularity, or revisions [25]. Furthermore, in a cost-utility analysis comparing rhinoplasty using costal cartilage autograft and homologous graft in the United States, the cost of rhinoplasty without hospitalization was similar [26]. However, with the complications associated with harvesting rib cartilage, the cost increased up to $21,099 [26]. This study shows that the upper limit of cost for rhinoplasty using homologous graft can be lower than that of rhinoplasty using autologous rib cartilage. However, the fact that their lower limits are similar sheds light on the need for a cheaper nasal implant material to be developed to decrease the lower limit of the cost.
In response to this demand, there have been many studies testing various xenograft materials for nasal reconstruction and rhinoplasty, such as porcine dermal collagen [13], equine pericardium [16], porcine small intestine submucosa [15], and bovine [27,28], porcine [29,30], and caprine [31] cartilages. Of the several xenograft tissues, decellularized cartilage matrix use has been studied, especially in orthopedic surgery for osteochondral regeneration [32] and plastic surgery for facial reconstruction [31]. Although the cartilage is speculated to be immunologically privileged because of its limited vascular, lymphatic, and neural supply, xenografts have the risk of infection and disease transmission [33,34]. More so, proper processing is needed to maintain its biochemical properties over time [35]. The 1980s and 1990s, the bovine irradiated glutaraldehyde preserved cartilage (Chondroplast) was used for facial reconstruction, but it had a high rate of graft loss due to resorption and infection [12].
However, with the development of decellularization and sterilization techniques, some studies have been testing decellularized cartilage xenograft as an implant material for rhinoplasty. Bhattacharya et al. [31] conducted a human study with decellularized caprine conchal cartilage in nine rhinoplasty and six microtia patients. Out of 15 patients, infection was observed, and xenograft was extruded in one patient each. Nonetheless, the operation time, average blood loss, and postoperative pain were significantly less in the xenograft group compared to those in the autograft group. Lin et al. [29] conducted in vitro and in vivo (rabbit model) experiments with decellularized porcine costal cartilage xenograft as the nasal implant. The study reported that it showed excellent biocompatibility and biosecurity. In addition, the imaging and histologic assessment of the retrieved xenograft exhibited a low degradation rate and ability to maintain its morphology. Herein, the efficacy and safety of decellularized equine cartilage for rhinoplasty were assessed and compared to the outcome of rhinoplasty with silicone implants. Although autograft is the gold-standard option for nasal implants, alloplastic silicone implant is most commonly used for dorsal augmentation in Asian rhinoplasty [11]. Hence, it was used for the control group in this study.
For objective evaluation, the nasofrontal angle and height of the nasion were measured and compared between the two groups. According to the position of the implant, the change in the postoperative nasofrontal angle and height of the nasion can vary [36,37]. The correction of the low radix is an important element of Asian rhinoplasty [37]; hence, both of them significantly increased in both groups, and there was no statistical difference. The statistically significant increase in these postoperative measurements reflects the effective dorsal augmentation of each implant. In this study, the change in nasofrontal angle and height of the nasion was also tracked 12 months after surgery. It verifies that the dorsal augmentation effect of equine cartilage and silicone implant remains 1 year after surgery and reflects that the resorption rate of equine cartilage is not significant.
This study has a few limitations. First, though this study showed significant surgical outcomes and safety, it can be posed that the result is less convincing due to a small number of cohorts and a relatively short follow-up period. Second, the absence of in vitro or in vivo animal studies regarding this study also comprises a limitation of this study. In addition, though measurements were taken through photographs before and after surgery, the lack of imaging study tool such as ultrasound that allows quantitative comparison is another limitation.
The ideal nasal implant needs to have abundant availability, affordable price, favorable efficacy, and safety. In this clinical study, the processed equine cartilage produced long-lasting results and high patient satisfaction as a nasal implant material. In addition, it has the advantage of providing good support because it is hyaline cartilage. The ease of carving is also an advantage. Due to the heterogeneity of the patient population, surgical procedure, and follow-up schedule, the study design for the head-to-head comparison of complication rate of various implant materials has many technical difficulties. Despite the relatively small patient population, this study showed that the equine cartilage could be used safely without any unrecoverable or serious adverse events. Through the non-inferiority test, the study also verified that the efficacy of MegaCartilage-E in rhinoplasty is not inferior to that of silicone implants. However, though it was not a significant difference, the result that the malposition of the implant was higher in the study group than in the control group needs further study.
Notes
Conflict of interest
This study was supported by L&C Bio (Suntechcity suite #605, #606, #607, 474; Seongnam, Korea) by sourcing nasal implants and funding study process. Jong Woo Choi is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Ethical approval
The study was approved by the Institutional Review Boards of Kangdong Sacred Heart Hospital (IRB No. 2018-12-001) and Ulsan University College of Medicine (IRB No. 2019–1162) and performed in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained.
Patient consent
The patient provided written informed consent for the publication and the use of her images.
Author contribution
Conceptualization: Jong Woo Choi, Yongho Shin, Chul Hoon Chung. Data curation: Hyunjong Yun, Jong Woo Choi, Joong Min Suh. Formal analysis: Chul Hoon Chung. Methodology: Yongho Shin, Chul Hoon Chung. Project administration: Yongjoon Chang, Woo Shik Jeong, Min Kyu Kang, Chul Hoon Chung. Visualization: Chul Hoon Chung. Writing - original draft: Yongjoon Chang, Hyunjong Yun, Jong Woo Choi, Joong Min Suh, Woo Shik Jeong, Hojin Park, Min Kyu Kang, Kuylhee Kim. Writing - review & editing: Yongjoon Chang, Hyunjong Yun, Joong Min Suh, Kuylhee Kim, Chul Hoon Chung. Investigation: Yongjoon Chang, Jong Woo Choi, Woo Shik Jeong, Hojin Park, Min Kyu Kang, Yongho Shin. Supervision: Yongjoon Chang, Kuylhee Kim, Chul Hoon Chung.
Abbreviations
ADE
adverse device events
CI
confidence interval
GAIS
Global Aesthetic Improvement Scale
mITT
modified intentionto-treat
PP
per-protocol
ROE
Rhinoplasty Outcome Evaluation
TEAE
treatment-emergent adverse event