|
 |
- Search
| Arch Craniofac Surg > Volume 23(5); 2022 > Article |
|
This article has been corrected. See "Correction: Omission of informed consent statements for the use of photographs, errors in describing consent for using photographs of minors, and absence of statements regarding conflict of interest of editorial board membership" in Volume 25 on page 159.
Abstract
Tongue reconstruction remains a major aspect of head and neck reconstructive procedures. Surgeons planning tongue reconstruction should consider several factors to optimize the overall outcomes. Specifically, various technical aspects related to tongue reconstruction have been found to affect the outcomes. Multidisciplinary teams dedicated to oncologic, reconstructive, and rehabilitative approaches play an essential role in the reconstructive process. Moreover, operative planning addressing certain patient-related and defect-related factors is crucial for optimizing functional speech and swallowing, as well as quality of life outcomes. Furthermore, tongue reconstruction is a delicate process, in which overall functional outcomes result from proper flap selection and shaping, recipient vessel preparation and anastomosis, surgical approaches to flap insetting, and postoperative management. The second part of this review summarizes these factors in relation to tongue reconstruction.
The first part of this review series on tongue reconstruction described functional aspects, starting with early reconstructive techniques through advances in flap options, including perforator flaps. The most crucial aspects required to optimize the functional outcomes of the reconstructive process were also summarized previously, as well as methods to analyze the effects of reconstruction and other outcomes, enabling better optimization in the future.
The second part of this review focuses on the technical aspects of tongue reconstruction, including defect-, patient-, and flap-specific factors. This is accompanied by a discussion of the adoption of new minimally invasive approaches and technologies to improve patients’ outcomes.
Recent advances in operative planning and microsurgical techniques have enabled precise, single-stage microsurgical reconstruction of the tongue [1]. Functional reconstruction of the tongue is the primary goal, and the meticulous execution of surgical plans optimizes patients’ outcomes. The diversity of surgical scenarios involved in tongue defects represents a confounding variable, requiring an optimum balance between ablative and reconstructive procedures. Surgical approaches to head and neck cancer involve either a single-team approach in which ablative and reconstructive procedures are performed by the same team, or a two-team approach in which the ablative and reconstructive procedures are performed by two separate teams [2]. Due to the complex surgical goals associated with tongue reconstruction, the adoption of the two-team approach resulted in a greater balance between ablation, enabling tumor resection with larger safety margins, and simultaneous reconstruction, involving specific flap selection to reconstruct wider defects and improve outcomes [2,3]. A retrospective evaluation of 2,968 patients who underwent both ablative and reconstructive head and neck interventions showed that most patients who underwent surgery using a single-team approach had smaller defects that were amenable for local flap coverage [3]. By contrast, the two-team approach was used for surgery in most patients with advanced surgical scenarios or severe comorbidities, who underwent glossectomy with or without concomitant procedures targeting adjacent structural defects (e.g., mandibulectomy) or required microsurgical flap reconstruction [3]. Operation times also varied, with some teams using a parallel model, consisting of simultaneous flap harvesting and tumor ablation, or a sequential model with flap harvest performed after the completion of tumor ablation. The parallel model has been expanded in certain situations (e.g., patients with defects requiring multiple flaps). In these situations, two reconstructive teams are involved at the same time throughout the procedure, regardless of whether the ablative team is also involved [3,4]. Thus, the operation time is dependent on either surgical model adopted and the complexity of the tongue defect. Factors that can alter the operation time include the need for harmony among surgical teams, meticulous planning, and surgeons’ familiarity with each other, all of which may improve overall patient outcomes [3]. Moreover, surgical teams should function within a multidisciplinary approach that include other specialties involved in the care of tongue cancer patients, such as oncologists, dietitians, nursing specialists, speech and language pathologists, and rehabilitation teams [5].
Our institution has utilized a two-team surgical model for the last 30 years, including an otorhinolaryngology ablative team and a plastic and reconstructive surgery team. These teams have worked well together and are also parts of a multidisciplinary team that includes speech, swallowing, occupational and rehabilitation specialists, and dietitians, along with other professionals, resulting in a much better quality of service and improved efficiency outcomes.
The tongue is the most frequent site of head and neck malignancies [6]. Regardless of the reconstructive option utilized, the success of flap reconstruction may depend on the proper choice of recipient neck vessels for microvascular anastomosis. The choice of recipient vessels may be affected by several factors, including demographic factors, such as patient age; comorbid conditions, such as diabetes and vascular diseases; risk factors, such as smoking and previous radiation therapy; and cancer-related factors, such as tumor location and type of neck dissection [6–10].
Various branches of the external carotid artery have shown success as recipient arteries. The superior thyroidal artery is most frequently utilized, followed by the facial and superficial temporal arteries and lastly lingual, transverse cervical, and hypoglossal arteries or the external carotid artery in an end-to-side fashion [6,11]. The superior thyroid artery has been associated with easier vessel dissection, enabling its simultaneous dissection during neck dissection, and positioning. However, small-diameter vessels (<1.5 mm) might be encountered, potentially presenting difficulties during micro-anastomosis, and this is an important factor to consider during flap selection [6,12].
Multiple options are available for venous anastomoses, such as the internal jugular vein and its side branches, as well as the superior thyroidal, facial, superficial temporal, lingual, hypoglossal veins and the external jugular, anterior jugular, and transverse cervical veins [6,13,14]. Using the internal jugular vein as a recipient site was shown to be advantageous, as this vein has a good caliber and multiple side branches that are easily accessible, allowing the vein to tolerate more than one anastomosis [13]. In comparison, the external jugular vein was associated with a slower flow rate and a smaller caliber; moreover, the external jugular vein was more easily compressible depending on the patient’s position, thereby increasing the risk of venous insufficiency [6]. Selecting the venous drainage should depend on the patient’s general status together with the surgeon’s experience with different techniques available for anastomosis. We have previously evaluated flap venous drainage patterns and found no significant differences among the selected veins in terms of venous congestion and flap survival [15]. By contrast, a prior history of neck radiation was associated with a significantly higher likelihood of venous congestion (odds ratio, 13.138; p<0.001) and a significantly lower rate of flap survival (odds ratio, 20.182; p=0.002) [15].
Certain flaps, such as the radial forearm free flap (RFFF), have the option of using a dual venous drainage system that might affect overall flap survival. An evaluation of outcomes of specific venous outflow patterns using single or dual venous anastomoses showed that dual anastomoses were associated with a significantly lower incidence of venous insufficiency, indicating that the use of two distinct venous drainage systems provides better outcomes. Furthermore, when selecting a single venous outflow, it is advisable to use the vein with the dominant venous backflow [16]. An example of head and neck vessel preparation as potential recipient vessels following dissection is shown in Fig. 1.
Previous radiation has been shown to have deleterious effects on neck vessels. Radiation alters the structural nature of the vessel walls, resulting in fibrin deposition, endothelial damage, and formation of microthrombi, and eventually leading to vessel occlusion [17]. These effects are related to the fractions and total dose of radiation and the time period between radiation and surgery [18,19]. The minimum dose of radiation found to affect neck vessels was found to be around 60 Gy [18,19]. In certain situations, radiation therapy with or without previous neck dissection creates a condition known as a “vessel-depleted neck,” in which proper vessels are unavailable for micro-anastomosis. In such situations, contralateral neck vessels should be used, followed by ipsilateral superficial temporal and lastly transverse cervical vessels, as concluded by one systematic review [20].
The selection of a recipient vessel is therefore challenging, as it can affect the overall success of head and neck reconstruction, especially in patients with other risk factors, such as previous surgery or radiation treatment. Preoperative planning based on radiological and non-radiological imaging such as computed tomography angiography (CTA) [21] has been shown to enable vessel selection. Recent advances in image processing and segmentation have allowed the three-dimensional (3D) volumetric reconstruction of CTA images, which have been shown to be valuable in visualizing recipient neck vessels and to facilitate the reconstructive approach [22]. An example of a 3D-reconstructed volumetric CTA image for a head and neck cancer patient is shown in Fig. 2.
Anastomosis may be end-to-end or end-to-side, with the choice dependent on whether arterial or venous anastomosis is being performed. End-to-end anastomosis is generally preferred for arterial anastomosis for several reasons [23]. For example, vessel mismatch up to 1:2 can be tolerated in the arteries but not in the veins due to the feasibility of arterial vessel dilatation. In addition, atherosclerotic plaques are frequently present in the walls of the carotid arteries, and these plaques are associated with vessel occlusion and thrombosis following end-to-side anastomosis. The presence of atherosclerotic plaques tends to decrease towards the distal branches of the main arteries. Furthermore, blood flow within end-to-side anastomoses is largely dependent on size of the arteriotomy; small openings possibly cause flow turbulence, whereas large openings might increase the blood flow to a level higher than the capacity of the venous drainage, leading to venous congestion. Finally, end-to-side anastomosis results in a relatively fixed point of attachment of the flap pedicle to the donor arteries. This may place the pedicle at risk of vessel occlusion or kinking, depending on the mobility of the patient’s neck [14]. In contrast to arterial anastomosis, both end-to-end and end-to-side anastomosis are feasible for venous anastomosis. Although veins are usually less tolerant of size mismatch than arteries, this deficiency of veins may be overcome by the availability of many recipient veins in the neck or the use of end-to-side anastomosis [24]. Furthermore, in necks lacking recipient veins, additional options include the use of contralateral neck vessels or the use of venous grafts [25,26].
Microvascular anastomosis is technically challenging and perhaps an essential part of the reconstructive approach. Regardless of the vessel alignment method utilized, the standard modality for micro-anastomosis has been the hand-sewing technique [27]. Apart from the technical demands, in order to optimize the chances of success of the hand-sewing technique, care must be taken to ensure proper vessel edge alignment with proper eversion of the edges to improve the patency rate and reduce the likelihood of thrombosis [28]. An example of flap anastomosis and positioning of the pedicle is shown in Fig. 3. Recent technical advances have enhanced the utility of mechanical anastomotic coupler devices, which have been shown to be a reliable, time-saving tool for microvascular anastomosis [29]. Mechanical coupler devices have been shown to be successful in head and neck reconstruction involving single or multiple veins and/or arteries, with good patency rates for lumen diameters of 1.5 to 3.5 mm for veins and 1 to 4 mm for arteries [28,30]. However, the use of these devices requires careful vessel selection and sufficient surgeon experience [31].
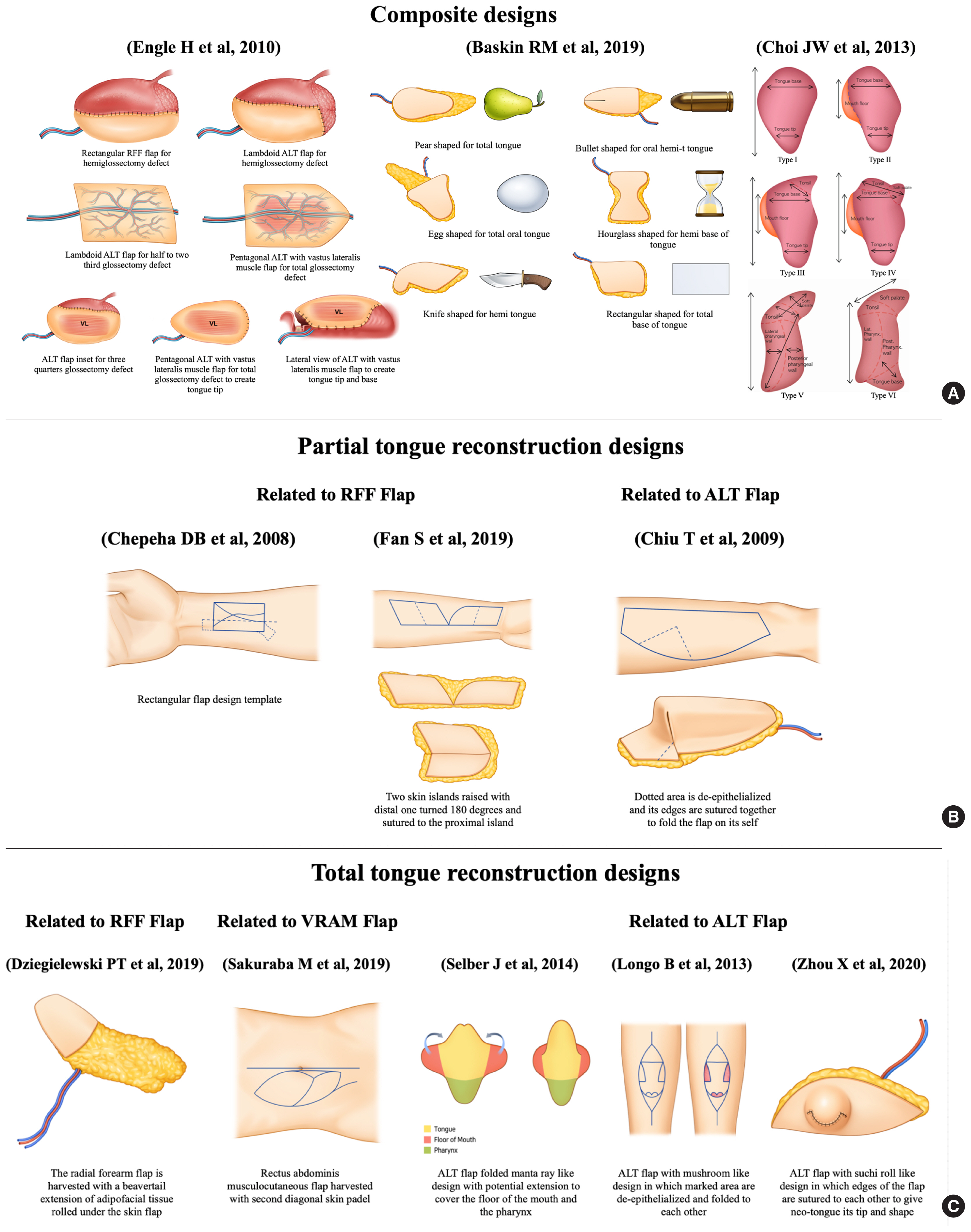
Flap selection for tongue defects should be tailored to individual patients, focusing on reconstructive plans that optimize functional outcomes. The shape of the flap variably associated with the final outcomes. Several flap designs and inset techniques have been designed to mimic the complex 3D structure of the tongue. As an example for hemi-glossectomy defects, omega-shaped RFFFs have a narrow waist that mimic the cross-sectional shape of the tongue [32]. Tongue tips have been optimized by rotating the flap of the remaining tip combined with a de-epithelialized skin segment to reduce pooling to the floor of the mouth and to improve tip sensation [33]. Furthermore, flap de-epithelialization and trimming together with a semi-circular design could deepen the sulcus between the neo-tongue and the floor of the mouth, thereby improving tongue elevation [34]. Moreover, a rectangular flap can be formed using a premade template constructed for a RFFF to establish a flap geometry with a proper definition of the boundaries between the neo-tongue and the floor of the mouth through a line of tension in the template [35]. Additionally, we have described our experience with a geometric multi-lobular flap design that addresses various tongue and tonsillar defects and can be formed by modifying an ovoid fasciocutaneous flap with the incorporation of multiple lobes [36]. The adjustable obtuse angle between the lobes allows flexibility in the flap inset, whereas an acute angle between the lobes was associated with difficulties in flap positioning during the inset process [36]. Flap designs are shown in Fig. 4A and B.
Various flap geometries have been described for total and subtotal tongue reconstruction. These flaps combine both bulk and mobility, which differ depending on the flap being used. The RFFFs used in reconstructive plans have different designs, with one incorporating a rectangular extension of the flap that resembles a beaver-tail. This beaver-tail extension of the RFFF has been utilized to cover adjacent soft tissue defects or de-epithelialized for volume augmentation [37,38]. One example of an anterolateral thigh (ALT) flap used for total and subtotal tongue reconstruction consisted of a mushroom-shaped ALT flap with the skin ellipse modified by multiple de-epithelialized skin islands, followed by flap folding to provide coverage of the floor of the mouth together with a protuberant neo-tongue [39]. Another modified ALT flap design consists of multiple skin ellipses sutured together to create a roll-shaped structure, called the “sushi roll technique,” resulting in a 3D structure with good bulk and protuberance [40]. In addition, a pentagonal musculocutaneous ALT flap with the V-shaped tip of the pentagon in a posterior position was shown to increase longitudinal tongue projection together with a sloping profile when viewed in a cross-section [11]. Moreover, a modified ALT flap, consisting of a template-based folded 3D design that resembled a manta-ray, was developed for complex defects that involve structures other than the tongue [41]. These flaps can be utilized for reconstruction and coverage of the total tongue, the floor of the mouth, and anterior pharyngeal defects. This technique was able to reproduce the native tongue dimensions together with its projection [41]. Other designs have been described for different flap options. For example, a vertical rectus abdominis musculocutaneous flap was utilized for total tongue reconstruction in a patient with a body mass index of less than 20 kg/m2. The flap was designed to have two skin islands, in which one was folded and provided the outer structure of the neo-tongue, and the other was de-epithelialized and provided the needed bulk of the reconstructed tongue [42]. The different flap designs mentioned in relation to the flap type and tongue defect type are presented in Fig. 4C.
Depending on the extent of tongue resection, a wide variety of flap designs have been developed for tongue reconstruction. Most of these flaps showed good functional outcomes, along with tongue mobility and protuberance. Due to the complexity of the clinical scenarios being managed, it is difficult to determine the relative superiority of these options, especially as most extant studies had a small sample size or utilized different methods to evaluate functional outcomes. In the technique we described, functional outcomes of ovoidal-multilobed fasciocutaneous flaps were compared with other series using patient-reported outcomes, with good speech and swallowing outcomes [36].
The choice of flaps also depends on demographic and behavioral factors, such as body habitus and body mass index, which may make it difficult to utilize certain flap designs, especially those for total and subtotal reconstruction. Subcutaneous tissue thickness might affect flap folding, which can compromise flap vascularity. Moreover, excessive bulk may limit tongue mobility, affecting the overall functional outcomes. These negative outcomes may be overcome by thickness-controlled perforator flap elevation, which makes it possible to harvest a flap of the desired thickness that can provide sufficient soft tissue without excessive bulkiness [43].
Virtual surgical planning and 3D printing have also been used to design personalized tongue-shaped flaps. For example, tumors visualized by preoperative 0.5-mm computed tomography scanning were initially analyzed in a 3D volumetric fashion to determine the extent of the tumor [44]. A tongue tumor-specific soft tissue cutting guide was subsequently designed and, based on the tumor data, a post-excisional defect guide was designed for flap harvesting. Although a proof-of-concept study showed that this framework was successful, additional studies are required to evaluate patient survival, functional outcomes, and cost-effectiveness.
The extent of surgery during oral cancer resection has also been associated with the success of flap inset. Surgical access frequently requires manipulation of the mandible. Although mandibulotomy accompanied by lip splitting provides wide exposure of the oral cavity [45,46], this method was associated with many complication related to wound healing, fistulas, and bony union issues [47,48]. The introduction of alternative mandible-preserving techniques, including mandibular sparing, mandibular lingual release, and transcervical, transpharyngeal, and transhyoid approaches, all of which preserve mandibular integrity, has made it possible to avoid these complications [47,49,50]. The utility of these modified approaches for tongue reconstruction was evaluated by determining oncologic safety and overall functional outcomes. Although these mandible/lip sparing techniques limit surgical exposure, they have survival profiles similar to that of mandibulotomy with lip splitting, as well as providing total disease control, safe surgical margins, and a lower rate of fistula formation [51]. Mandibular lingual release and pull-through resection were found to result in better facial appearance, improved quality of life, lower infection rates, and teeth viability [52,53]. Functional outcomes, such as speech and tongue movement, did not differ significantly in patients who underwent surgery using the mandible split and mandible preservation techniques [51]. Surgical access was more limited using mandible preservation techniques, whereas flap inset using these methods was improved through a logical plan involving the placement of key sutures at the most posterior and anterior extensions of the defect and flap inset prior to the micro-anastomoses. This facilitated pedicle placement during anastomosis without placing undue tension on the pedicle. Final flap inset required special care of suture placement at the location of the pedicle while it passes to the neck. Another technique included the placement of multiple unlocked suture threads at the edges of the tongue and floor of the mouth, followed by passing the threads to the flap in a sequential pattern while locking the sutures for final flap insertion; this method was called the “parachute technique” [54].
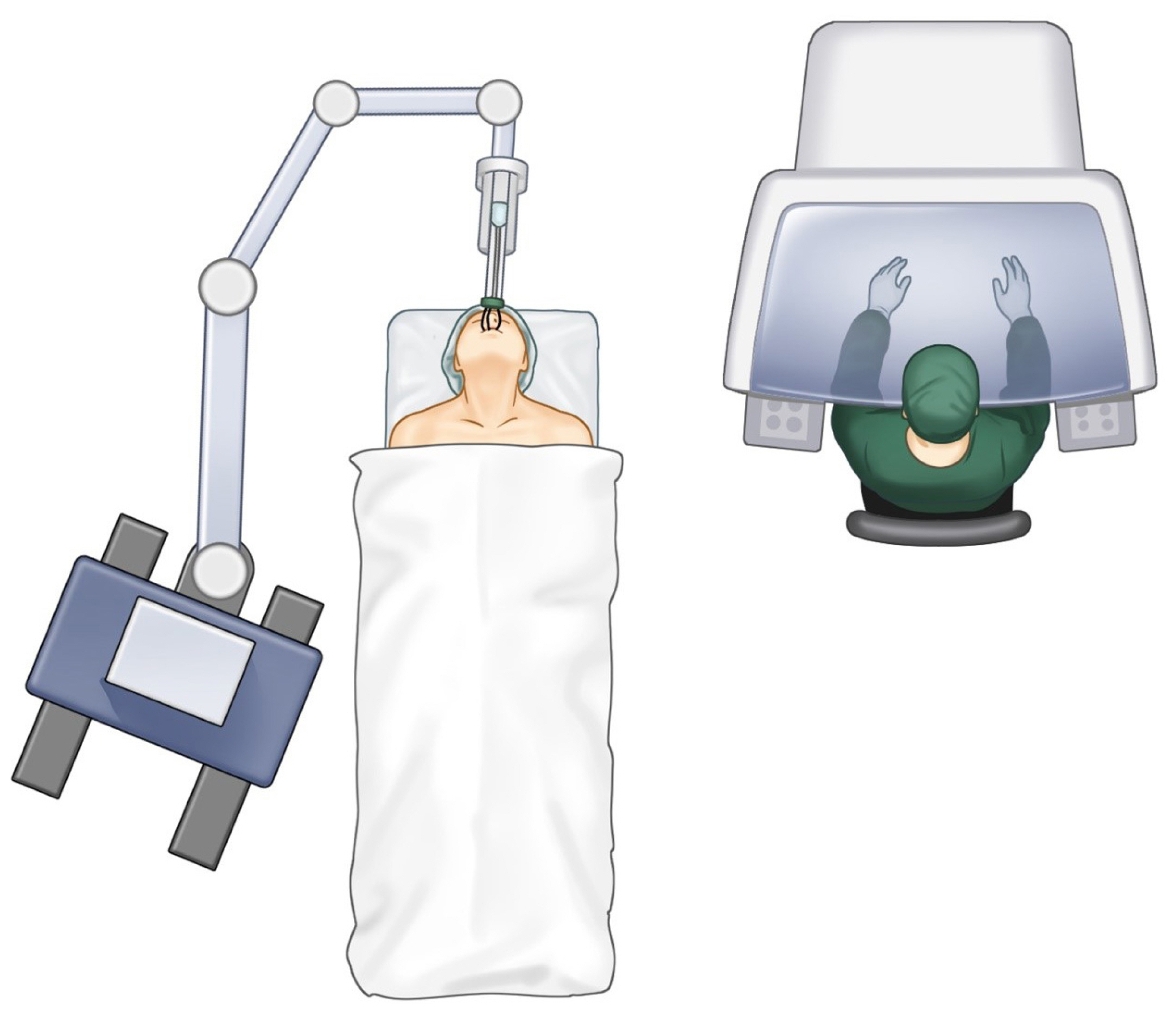
Recent advances in robotics and the integration of robotic-assisted minimally invasive approaches in various surgical disciplines have resulted in the utilization of robotics in the management of patients with head and neck cancer. Robotic surgery has allowed a minimally invasive approach with safety profiles similar to those of traditional surgical modalities [55]. The use of robotics in oropharyngeal cancer resection, a method called transoral robotic surgery (TORS) shown in Fig. 5, has allowed surgeons to approach complex structures with limited visualization, including the base of the tongue, without the need for traditional lip-split incision and/or mandibulotomy. Moreover, TORS was found to result in faster recovery times and improved cosmetic outcomes [56,57]. Further adaptations of robotic surgery have allowed concomitant neck dissection through the use of approaches, such as retro-auricular and modified face-lift incisions, as well as allowing a simultaneous approach to neck vessels [58,59]. TORS has mostly been used to manage patients with tongue base cancer, and it has been recommended for lesions involving less than 50% of the tongue base, tumors in which TORS can achieve negative resection margins, and tumors that can be adequately visualized and accessed by robot retractors in which conditions such as trismus and morbid obesity hinders such access [60]. TORS allows a wider view of the surgical field, together with better 3D visualization of structures and more comfortable access to the tumor. Furthermore, TORS enables the use of miniaturized instruments with tremor filtration and greater dexterity in confined spaces [61].
TORS for tongue reconstruction was initially performed on patients with small tongue base defects that were allowed to heal by secondary intention, while flap reconstruction was recommended for larger defects, without a clear delineation between these lesion types [62]. The extent of tongue base resection has been shown to affect the risk of aspiration. For example, patients with defects involving 0% to 25% of the tongue base were found to be at an 11.5% risk of aspiration, with a higher level of risk associated with an increased extent of resection [63]. Additionally, the initial use of TORS for robotic-assisted tongue reconstruction consisted of the use of robotics primarily for tumor ablation, whereas conventional methods of neck access, vessel exposure, and micro-anastomosis were utilized for reconstruction, with use of the robotic arms for flap insetting [64]. Further advances in TORS and robotic-assisted neck dissection, together with vessel exposure, allowed simultaneous robotic flap micro-anastomosis and insertion, with good success. In one study, five patients underwent successful robotic-assisted flap anastomosis and inset for oropharyngeal structure reconstruction, including reconstruction of the tongue and tongue base using RFFF and ALT flaps. The superior thyroidal artery was used for most of these patients. One technical difficulty associated with robotic-assisted micro-anastomosis in these patients was the need to view the blood vessels in an oblique fashion (about 45°) rather than the conventional 90° frontal view, with a second difficulty being in selecting a recipient vessel [65]. Furthermore, robot docking and port placement extended the operation time, emphasizing the importance of team ergonomics, with tasks performed in parallel. Additional studies have reported the feasibility and success of robotic-assisted microsurgery in patients with different types of head and neck cancers [66,67].
These findings indicate the need for plastic surgeons to become familiar with advanced micro-anastomosis techniques, as well as with improvements and adaptations of robotics in head and neck reconstruction. Among the drawbacks of robotics, apart from the increased operative time and the need for surgeons to become familiar with how to use these systems, was the lack of haptic feedback from the robot during the process of micro-anastomosis. Although this lack of feedback did not affect the patency rate after anastomosis in small series, additional investigations are required in larger numbers of patients, with surgery performed by operators with different levels of experience in the delicate handling of blood vessels [67].
Most studies assessing function after TORS tongue resection and reconstruction have reported swallowing outcomes, with special emphasis on healing by secondary intention. Functional swallowing and deglutition began to return at 3 months postoperatively, with complete return of preoperative functional levels at 1 year [68,69]. Most studies of flap reconstruction have reported technical aspects and costs, with brief descriptions of other factors such as tracheostomy and tube feeding [70]. Further investigations comparing speech and swallowing functions after robotic and traditional treatment techniques that include the tongue and other oropharyngeal structures involved in cancer resection are warranted.
Timely multidisciplinary treatment focused on improving functional swallowing, speech, and quality of life is an essential factor contributing to proper functional recovery after tongue resection and reconstruction.
Dysphagia and dysarthria are two common problems experienced by many tongue cancer patients, starting during the preoperative period and continuing postoperatively, regardless of the reconstructive technique applied [71,72]. Proper patient counseling about these conditions is recommended, starting during the preoperative period, since counseling increases the likelihood of strict compliance with the rehabilitative plan and promotes ultimate functional recovery [73]. The optimal time to assess swallowing is variable, with earlier interventions regarded as superior to later interventions, primarily because fibrosis leading to increased swallowing dysfunction that occurs primarily during the first 3 months after cancer treatment, especially in patients receiving radiation therapy [74,75]. The optimal timing of speech assessment is similar to that of swallowing, as an earlier evaluation helps speech and language therapists to design treatments to manage patients’ voices and any associated resonances [76,77]. Strict adherence to swallowing and speech rehabilitative plans was found to strongly correlate with functional recovery, with most patients recovering good speech and swallowing function 1 year after surgery, although recovery times can vary depending on the surgical defect and treatment plan [72,78].
A factor that might affect functional recovery during the postoperative period is the postoperative placement of a tracheostomy to secure the airway. Most patients who underwent total and subtotal glossectomy and flap reconstruction were decannulated at a median of 15 days postoperatively [79]. The rates of tube feeding, poorer speech outcomes, and prolonged swallowing rehabilitation were found to be higher in patients with tracheostomy than those without tracheostomy, with decannulation providing improvements [71,72].
A long-term individualized follow-up plan should be instituted for each patient, with the main focus being strict compliance with the rehabilitative plan, quality of life improvements, abstinence from smoking and alcohol drinking, dental and oral hygiene, and most importantly, early identification of locoregional recurrence [78].
The tongue is a vital structure that is crucial for various daily living activities and quality of life. Tongue reconstruction requires a delicate understanding of the tongue together with a team-oriented approach to maximize reconstructive functional outcomes. These plans start with the initial encounter with the patient through an extended postoperative period, thereby optimizing survival and functional recovery. Because the tongue is a mobile and complex 3D structure, replacing it with similar tissue requires meticulous reconstruction, ensuring both flap survival and a mobile, protuberant tongue. Technical advances have allowed minimally invasive techniques that are already shaping the future directions of tongue reconstruction. Additional investigations of these minimally invasive techniques are required to address many aspects related to proper functional outcomes.
Notes
Conflict of interest
Jong-Woo Choi is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Fig. 1
Preparation of head and neck recipient vessels after the completion of neck dissection. In this example, the facial and superior thyroidal vessels, together with the internal jugular vein, were prepared.

Fig. 2
Preoperative images. Three-dimensional reconstructed volumetric computed tomography angiography images showing the visualization of many recipient vessels in (A) arterial phase and (B) venous phase.
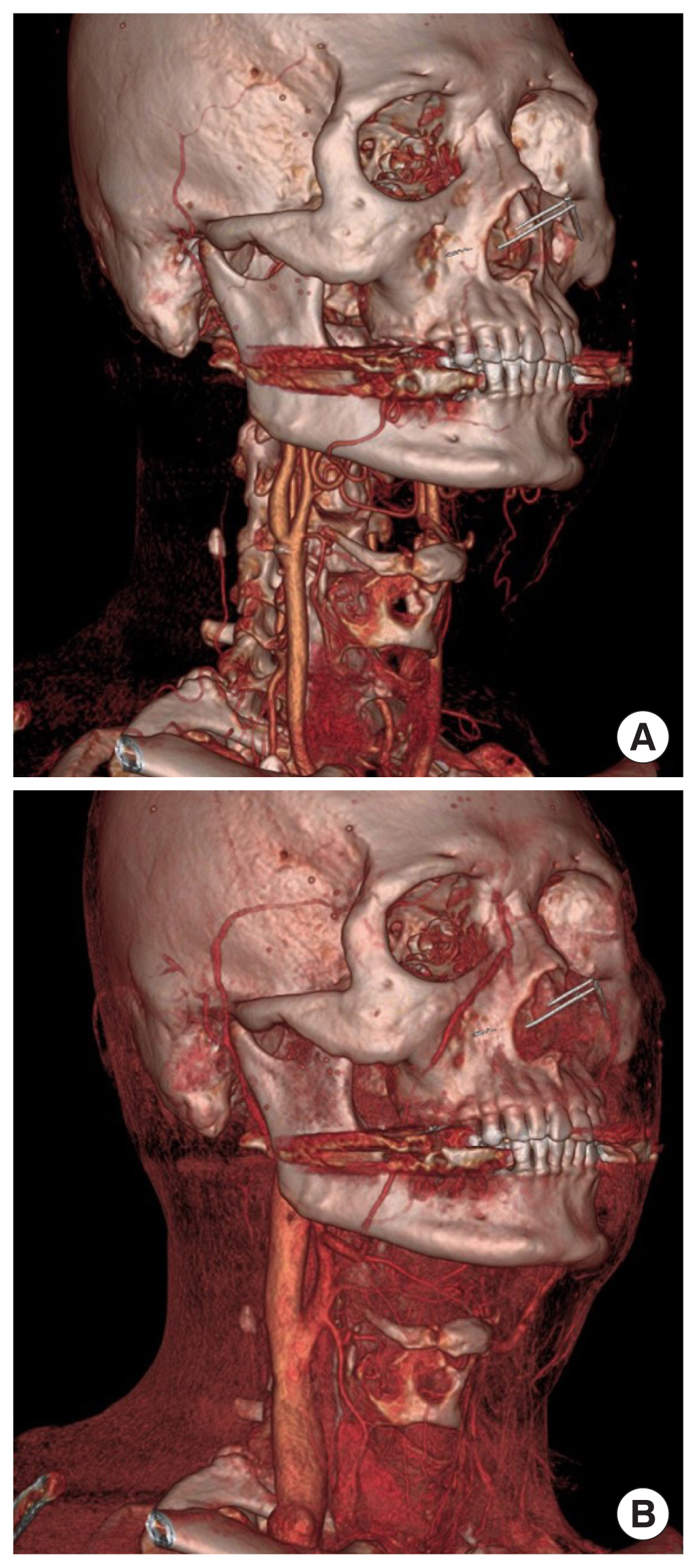
Fig. 3
Intraoperative photographs of flap micro-anastomosis. (A) The flap pedicle following anastomoses to the recipient superior thyroidal artery and the recipient side branch of the internal jugular vein. (B) Final position of the pedicle prior to skin closure after ensuring no kinking of the pedicle.
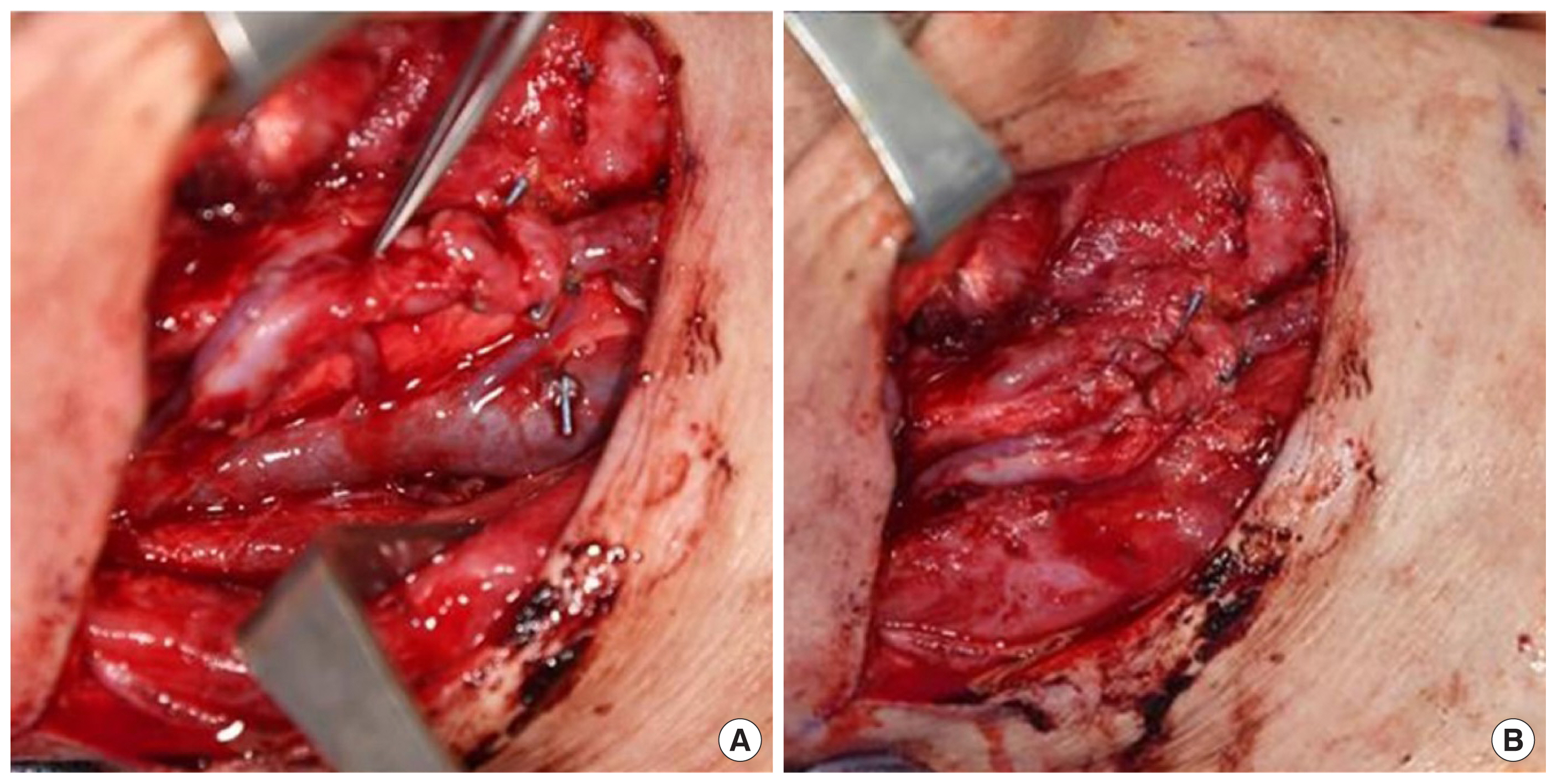
Fig. 4
Different flap designs in relation to flap type and defect type. (A) The composite designs described by several authors incorporate certain geometric features depending on the flap type and extent of the tongue resection defect. (B) Summary of the different designs described for partial tongue reconstruction in relation to both the radial forearm free (RFF) flap and anterolateral thigh (ALT) flap. (C) The geometric designs that have been described for total and subtotal tongue resection defects with the incorporation of RFF, ALT, and vertical rectus abdominis musculocutaneous (VRAM) flaps.
REFERENCES
1. Varkey P, Liu YT, Tan NC. Multidisciplinary treatment of head and neck cancer. Semin Plast Surg 2010;24:331-4.



2. Ghazizadeh S, Kuan EC, Mallen-St Clair J, Abemayor E, Luu Q, Nabili V, et al. It takes two: one resects, one reconstructs. Otolaryngol Clin North Am 2017;50:747-53.


3. Torabi SJ, Chouairi F, Dinis J, Alperovich M. Head and neck reconstructive surgery: characterization of the one-team and two-team approaches. J Oral Maxillofac Surg 2020;78:295-304.


4. Wallace CG, Tsao CK, Wei FC. Role of multiple free flaps in head and neck reconstruction. Curr Opin Otolaryngol Head Neck Surg 2014;22:140-6.


5. Jeong WS, Oh TS. Oral and oropharyngeal reconstruction with a free flap. Arch Craniofac Surg 2016;17:45-50.



6. Chung JH, Kim KJ, Jung KY, Baek SK, Park SH, Yoon ES. Recipient vessel selection for head and neck reconstruction: a 30-year experience in a single institution. Arch Craniofac Surg 2020;21:269-75.




7. Serletti JM, Higgins JP, Moran S, Orlando GS. Factors affecting outcome in free-tissue transfer in the elderly. Plast Reconstr Surg 2000;106:66-70.


8. Bengtson BP, Schusterman MA, Baldwin BJ, Miller MJ, Reece GP, Kroll SS, et al. Influence of prior radiotherapy on the development of postoperative complications and success of free tissue transfers in head and neck cancer reconstruction. Am J Surg 1993;166:326-30.


9. Yagi S, Suyama Y, Fukuoka K, Takeuchi H, Kitano H. Recipient vessel selection in head and neck reconstruction based on the type of neck dissection. Yonago Acta Med 2016;59:159-62.


10. Suh JM, Chung CH, Chang YJ. Head and neck reconstruction using free flaps: a 30-year medical record review. Arch Craniofac Surg 2021;22:38-44.




11. Engel H, Huang JJ, Lin CY, Lam W, Kao HK, Gazyakan E, et al. A strategic approach for tongue reconstruction to achieve predictable and improved functional and aesthetic outcomes. Plast Reconstr Surg 2010;126:1967-77.


12. Chen YC, Scaglioni MF, Huang EY, Kuo YR. Utility of “open-Y” anastomosis technique in the use of superior thyroid artery as recipient vessel for head and neck reconstruction with free flap. Microsurgery 2016;36:391-6.



13. Yamamoto Y, Nohira K, Kuwahara H, Sekido M, Furukawa H, Sugihara T. Superiority of end-to-side anastomosis with the internal jugular vein: the experience of 80 cases in head and neck microsurgical reconstruction. Br J Plast Surg 1999;52:88-91.


14. Chia HL, Wong CH, Tan BK, Tan KC, Ong YS. An algorithm for recipient vessel selection in microsurgical head and neck reconstruction. J Reconstr Microsurg 2011;27:47-56.


15. Choi JW, Kim YC, Jeon DN, Jeong WS, Koh KS, Oh TS, et al. Impact of recipient vein selection on venous patency and free flap survival in 652 head and neck reconstructions. J Reconstr Microsurg 2020;36:73-81.


16. Kim YC, Kim MJ, Kim HB, Kim SC, Choi JW. Impact of venous outflow pattern on flap compromise in head and neck reconstruction: review of 309 radial forearm free flaps. J Craniofac Surg 2019;30:1194-7.


17. Guelinckx PJ, Boeckx WD, Fossion E, Gruwez JA. Scanning electron microscopy of irradiated recipient blood vessels in head and neck free flaps. Plast Reconstr Surg 1984;74:217-26.


18. Ho AL, Lyonel Carre A, Patel KM. Oncologic reconstruction: general principles and techniques. J Surg Oncol 2016;113:852-64.



19. Abouyared M, Katz AP, Ein L, Ketner J, Sargi Z, Nicolli E, et al. Controversies in free tissue transfer for head and neck cancer: a review of the literature. Head Neck 2019;41:3457-63.



20. Kushida-Contreras BH, Manrique OJ, Gaxiola-Garcia MA. Head and neck reconstruction of the vessel-depleted neck: a systematic review of the literature. Ann Surg Oncol 2021;28:2882-95.



21. Smit JM, Dimopoulou A, Liss AG, Zeebregts CJ, Kildal M, Whitaker IS, et al. Preoperative CT angiography reduces surgery time in perforator flap reconstruction. J Plast Reconstr Aesthet Surg 2009;62:1112-7.


22. Kwon JG, Hong DW, Choi JW. Clinical applications of augmented reality technology in microsurgical planning of head and neck reconstruction. J Craniofac Surg 2022;33:863-6.


23. Nahabedian MY, Singh N, Deune EG, Silverman R, Tufaro AP. Recipient vessel analysis for microvascular reconstruction of the head and neck. Ann Plast Surg 2004;52:148-55.


24. Tan BK, Wong CH, Chew W, Hong SW. Use of the slit arteriotomy for end-to-side arterial anastomosis in free-tissue transfers to the extremities. J Plast Reconstr Aesthet Surg 2009;62:1519-23.


25. Yazar S. Selection of recipient vessels in microsurgical free tissue reconstruction of head and neck defects. Microsurgery 2007;27:588-94.



26. Cheng HT, Lin FY, Chang SC. Evidence-based analysis of vein graft interposition in head and neck free flap reconstruction. Plast Reconstr Surg 2012;129:853e-854e.


27. Zhang T, Lubek J, Salama A, Caccamese J, Coletti D, Dyalram D, et al. Venous anastomoses using microvascular coupler in free flap head and neck reconstruction. J Oral Maxillofac Surg 2012;70:992-6.


28. Assoumane A, Wang L, Liu K, Shang ZJ. Use of couplers for vascular anastomoses in 601 free flaps for reconstruction of defects of the head and neck: technique and two-year retrospective clinical study. Br J Oral Maxillofac Surg 2017;55:461-4.


29. Frederick JW, Sweeny L, Carroll WR, Rosenthal EL. Microvascular anastomotic coupler assessment in head and neck reconstruction. Otolaryngol Head Neck Surg 2013;149:67-70.




30. Thorpe E, Patil Y. Mechanical venous anastomosis in head and neck microvascular reconstruction as an equivalent to the gold standard. Ear Nose Throat J 2017;96:E32-6.



31. Rozen WM, Whitaker IS, Acosta R. Venous coupler for free-flap anastomosis: outcomes of 1,000 cases. Anticancer Res 2010;30:1293-4.

32. Hsiao HT, Leu YS, Lin CC. Tongue reconstruction with free radial forearm flap after hemiglossectomy: a functional assessment. J Reconstr Microsurg 2003;19:137-42.


33. Davison SP, Grant NN, Schwarz KA, Iorio ML. Maximizing flap inset for tongue reconstruction. Plast Reconstr Surg 2008;121:1982-5.


35. Chepeha DB, Teknos TN, Shargorodsky J, Sacco AG, Lyden T, Prince ME, et al. Rectangle tongue template for reconstruction of the hemiglossectomy defect. Arch Otolaryngol Head Neck Surg 2008;134:993-8.


36. Choi JW, Lee MY, Oh TS. The application of multilobed flap designs for anatomic and functional oropharyngeal reconstructions. J Craniofac Surg 2013;24:2091-7.


37. Seikaly H, Rieger J, O’Connell D, Ansari K, Alqahtani K, Harris J. Beavertail modification of the radial forearm free flap in base of tongue reconstruction: technique and functional outcomes. Head Neck 2009;31:213-9.


38. Dziegielewski PT, Rieger J, Shama MA, O’Connell DA, Harris JR, Seikaly H. Beavertail modification of the radial forearm free flap in total oral glossectomy reconstruction: technique and functional outcomes. Oral Oncol 2019;96:71-6.


39. Longo B, Pagnoni M, Ferri G, Morello R, Santanelli F. The mushroom-shaped anterolateral thigh perforator flap for subtotal tongue reconstruction. Plast Reconstr Surg 2013;132:656-65.


40. Zhou X, He ZJ, Su YX, Zhang S, Gong ZJ, Wu HJ. “Sushi roll” technique for precise total tongue functional reconstruction using a pre-sutured femoral anterolateral myocutaneous flap. Oral Oncol 2020;110:104866.


41. Selber JC, Robinson J. The manta ray flap: a technique for total glossectomy reconstruction. Plast Reconstr Surg 2014;134:341e-344e.

42. Sakuraba M, Asano T, Miyamoto S, Hayashi R, Yamazaki M, Miyazaki M, et al. A new flap design for tongue reconstruction after total or subtotal glossectomy in thin patients. J Plast Reconstr Aesthet Surg 2009;62:795-9.


43. Choi JW, Park JS, Kim JH. Extensive facial reconstruction using thickness-controlled perforator free flaps. Plast Reconstr Surg Glob Open 2020;8:e3210.



44. Koumoullis H, Burley O, Kyzas P. Patient-specific soft tissue reconstruction: an IDEAL stage I report of hemiglossectomy reconstruction and introduction of the PANSOFOS flap. Br J Oral Maxillofac Surg 2020;58:681-6.


45. Shah JP, Gil Z. Current concepts in management of oral cancer: surgery. Oral Oncol 2009;45:394-401.



46. Satpathy S, Dam A, Hossain MA, Chatterjee J. Double mandibular osteotomy with segmental mandibular swing approach to parapharyngeal space. Natl J Maxillofac Surg 2014;5:213-6.



47. Scheithauer MO, Bohn JC, Riechelmann H. Median sagittal mandibulotomy in head-neck tumors. Laryngorhinootologie 2000;79:490-7.


48. Dziegielewski PT, Mlynarek AM, Dimitry J, Harris JR, Seikaly H. The mandibulotomy: friend or foe? Safety outcomes and literature review. Laryngoscope 2009;119:2369-75.



49. Holsinger FC, Laccourreye O, Weber RS. Surgical approaches for cancer of the oropharynx. Oper Tech Otolayngol Head Neck Surg 2005;16:40-8.

50. Civantos F, Wenig BL. Transhyoid resection of tongue base and tonsil tumors. Otolaryngol Head Neck Surg 1994;111:59-62.



51. Pang P, Li RW, Shi JP, Xu ZF, Duan WY, Liu FY, et al. A comparison of mandible preservation method and mandibulotomy approach in oral and oropharyngeal cancer: a meta-analysis. Oral Oncol 2016;63:52-60.


52. Cheng SJ, Ko HH, Lee JJ, Kok SH. Comparison of long-term outcomes between pull-through resection and mandibular lip-split surgery for T4a tongue/floor of mouth cancers. Head Neck 2018;40:144-53.



53. Li H, Li J, Yang B, Su M, Xing R, Han Z. Mandibular lingual release versus mandibular lip-split approach for expanded resection of middle-late tongue cancer: a case-control study. J Craniomaxillofac Surg 2015;43:1054-8.


54. Liu CJ, Fang KH, Chang CC, Lin ET, Chang GH, Shen JH, et al. Application of “parachute” technique for free flap reconstruction in advanced tongue cancer after ablation without lip-jaw splitting: a retrospective case study. Medicine (Baltimore) 2019;98:e16728.


55. Aubry K, Yachine M, Perez AF, Vivent M, Lerat J, Scomparin A, et al. Transoral robotic surgery for head and neck cancer: a series of 17 cases. Eur Ann Otorhinolaryngol Head Neck Dis 2011;128:290-6.


56. O’Malley BW Jr, Weinstein GS, Snyder W, Hockstein NG. Transoral robotic surgery (TORS) for base of tongue neoplasms. Laryngoscope 2006;116:1465-72.


57. Genden EM, Desai S, Sung CK. Transoral robotic surgery for the management of head and neck cancer: a preliminary experience. Head Neck 2009;31:283-9.


58. Kowalski LP, Lira RB. Anatomy, technique, and results of robotic retroauricular approach to neck dissection. Anat Rec (Hoboken) 2021;304:1235-41.



59. Park YM, Lee WJ, Yun IS, Lee DW, Lew DH, Lee JM, et al. Free flap reconstruction after robot-assisted neck dissection via a modified face-lift or retroauricular approach. Ann Surg Oncol 2013;20:891-8.



60. Park ES, Shum JW, Bui TG, Bell RB, Dierks EJ. Robotic surgery: a new approach to tumors of the tongue base, oropharynx, and hypopharynx. Oral Maxillofac Surg Clin North Am 2013;25:49-59.

61. Mercante G, Ruscito P, Pellini R, Cristalli G, Spriano G. Transoral robotic surgery (TORS) for tongue base tumours. Acta Otorhinolaryngol Ital 2013;33:230-5.


62. de Almeida JR, Park RC, Genden EM. Reconstruction of transoral robotic surgery defects: principles and techniques. J Reconstr Microsurg 2012;28:465-72.


63. Smith JE, Suh JD, Erman A, Nabili V, Chhetri DK, Blackwell KE. Risk factors predicting aspiration after free flap reconstruction of oral cavity and oropharyngeal defects. Arch Otolaryngol Head Neck Surg 2008;134:1205-8.


64. Meccariello G, Montevecchi F, Sgarzani R, Vicini C. Defect-oriented reconstruction after transoral robotic surgery for oropharyngeal cancer: a case series and review of the literature. Acta Otorhinolaryngol Ital 2018;38:569-74.



65. Song HG, Yun IS, Lee WJ, Lew DH, Rah DK. Robot-assisted free flap in head and neck reconstruction. Arch Plast Surg 2013;40:353-8.



66. Chalmers R, Schlabe J, Yeung E, Kerawala C, Cascarini L, Paleri V. Robot-assisted reconstruction in head and neck surgical oncology: the evolving role of the reconstructive microsurgeon. ORL J Otorhinolaryngol Relat Spec 2018;80:178-85.



67. Lai CS, Lu CT, Liu SA, Tsai YC, Chen YW, Chen IC. Robot-assisted microvascular anastomosis in head and neck free flap reconstruction: preliminary experiences and results. Microsurgery 2019;39:715-20.



68. Mercante G, Masiello A, Sperduti I, Cristalli G, Pellini R, Spriano G. Quality of life and functional evaluation in patients with tongue base tumors treated exclusively with transoral robotic surgery: a 1-year follow-up study. J Craniomaxillofac Surg 2015;43:1561-6.


69. Salmon KM, Ruiz C, Cognetti DM, Curry JM, Luginbuhl AJ, Bar-Ad V, et al. Functional swallow-related outcomes following transoral robotic surgery for base of tongue carcinoma. Dysphagia 2022;37:28-36.



70. Biron VL, O’Connell DA, Barber B, Clark JM, Andrews C, Jeffery CC, et al. Transoral robotic surgery with radial forearm free flap reconstruction: case control analysis. J Otolaryngol Head Neck Surg 2017;46:20.




71. Manrique OJ, Leland HA, Langevin CJ, Wong A, Carey JN, Ciudad P, et al. Optimizing outcomes following total and subtotal tongue reconstruction: a systematic review of the contemporary literature. J Reconstr Microsurg 2017;33:103-11.


72. Vincent A, Kohlert S, Lee TS, Inman J, Ducic Y. Free-flap reconstruction of the tongue. Semin Plast Surg 2019;33:38-45.



73. Chen DW, Wang T, Shey-Sen Ni J, Sandulache VC, Graboyes EM, Worley M, et al. Prognostic factors associated with achieving total oral diet after glossectomy with microvascular free tissue transfer reconstruction. Oral Oncol 2019;92:59-66.



74. Murphy BA, Gilbert J. Dysphagia in head and neck cancer pa tients treated with radiation: assessment, sequelae, and rehabilitation. Semin Radiat Oncol 2009;19:35-42.


75. Logemann JA, Pauloski BR, Rademaker AW, Lazarus CL, Gaziano J, Stachowiak L, et al. Swallowing disorders in the first year after radiation and chemoradiation. Head Neck 2008;30:148-58.



76. Karlsson T, Johansson M, Andrell P, Finizia C. Effects of voice rehabilitation on health-related quality of life, communication and voice in laryngeal cancer patients treated with radiotherapy: a randomised controlled trial. Acta Oncol 2015;54:1017-24.


77. van Gogh CD, Verdonck-de Leeuw IM, Langendijk JA, Kuik DJ, Mahieu HF. Long-term efficacy of voice therapy in patients with voice problems after treatment of early glottic cancer. J Voice 2012;26:398-401.


- TOOLS
-
METRICS

-
- 2 Crossref
- Scopus
- 3,322 View
- 142 Download
- Related articles in ACFS
-
Current status and evolution of microsurgical tongue reconstructions, part I2022 August;23(4)