 |
 |
- Search
| Arch Craniofac Surg > Epub ahead of print |
|
Abstract
A 59-year-old woman presented to our clinic with a 3.5× 3-cm protruding mass on her forehead. A skull X-ray revealed a radiolucent osteolytic lesion on the left side of the frontal bone. Additionally, computed tomography showed a 3.1× 1.7× 3.6-cm mass exhibiting a “sunburst” pattern situated between the outer and inner tables of the skull, just superior and lateral to the left frontal sinus. This pattern suggested the presence of an intraosseous vascular malformation (IVM). The lesion was approached via a bicoronal incision. En-bloc resection was performed, removing the mass along with approximately 0.5 cm of the surrounding normal bone without injury to the exposed frontal sinus mucosa. The exposed mucosa was reinforced with a galeal flap, and cranioplasty with bone cement was performed to repair the resulting bony defect. Pathological examination confirmed a diagnosis of intraosseous cavernous-type malformation with mixed cavernous and capillary histological features. We report this case of IVM and review the existing literature, highlighting the satisfactory functional and aesthetic outcomes after surgery.
Intraosseous vascular malformation (IVM) is a rare, benign, slow-growing skeletal tumor that can occur at any age but is more commonly seen in middle-aged individuals, with the highest incidence in the fourth decade of life. IVM most frequently affects the vertebral column; in contrast, IVM of the skull is relatively rare, representing only 0.2% of all primary benign cranial tumors [1]. IVM was first characterized in the medical literature by Toynbee in 1845 [2], who described vascular tumors developing within the bone substance. The first operation performed to treat such a tumor was reported in 1894 by Pilcher [3].
The frontal bone is the cranial site most frequently affected by IVM, followed by the parietal bone [4]. The pathogenesis of this condition remains unclear, but it is thought to be either congenital or associated with prior trauma [5,6]. In this report, we present a case of IVM of the frontal bone, specifically involving the bony component of the frontal sinus. The lesion was completely removed without injury to the frontal sinus mucosa.
A 59-year-old woman presented to our clinic with a 3.5× 3-cm protruding mass on her forehead (Fig. 1). The mass was painess and slow-growing, and the patient displayed no other symptoms, including neurological deficits. The mass was hard, immobile, non-pulsatile, and non-tender, with the overlying skin appearing normal and similar in color to the surrounding facial skin. The patient had no history of head trauma. The mass had appeared spontaneously 10 years prior and had grown over time without treatment. Skull X-ray revealed a radiolucent osteolytic lesion on the left side of the frontal bone. Additionally, computed tomography (CT) showed a 3.1× 1.7× 3.6-cm mass with a “sunburst” pattern situated between the outer and inner tables of the skull and located superior and lateral to the left frontal sinus. These findings were indicative of an IVM (Fig. 2). Consequently, we collaborated with a neurosurgery team for surgical treatment. The lesion was approached via a bicoronal incision, and en-bloc resection was performed with a margin of approximately 0.5 cm of normal bone. Due to the proximity of the lesion to the left frontal sinus, exposure of the sinus mucosa was unavoidable, but this structure was not injured (Fig. 3A). The exposed mucosa was reinforced with a galeal flap (Fig. 3B). To address the resulting 4× 4.1-cm bone defect, cranioplasty was performed using bone cement (Fig. 3C). Pathological examination confirmed a diagnosis of intraosseous cavernous-type malformation with mixed cavernous and capillary histological features (Fig. 4). Throughout 6 months of follow-up, the patient showed no cosmetic impairment and displayed no clinical or radiological signs of recurrence (Fig. 1B).
IVM is a rare type of bone tumor, representing 0.7% to 1.0% of all bone tumors. These tumors are most commonly located in the vertebral column and are infrequently observed in the skull. IVMs tend to be observed in the fourth decade of life and exhibit a female predominance, with a male-to-female ratio of 1:1.67. Within the skull, the frontal bone is the most frequently affected, followed by the parietal and occipital bones [4].
The exact pathogenesis of IVM remains elusive, but it is thought to involve a combination of genetic factors and anomalies in angiogenesis. In one report, Vargel et al. [5] indicated that their cases were associated with specific genetic mutations. For instance, mutations in the cerebral cavernous malformation (CCM) genes CCM1, CCM2, or CCM3 have been linked to CCMs, which may also involve IVMs. These genes play a role in the regulation of blood vessel formation and the maintenance of endothelial cell integrity. Separately, Yu et al. [6] suggested that IVM may be related to prior trauma or injury to the affected bone. This theory posits that trauma disrupts the normal vascular architecture, leading to the development of cavernous malformations within the bone. However, recent cases of IVM with no history of trauma challenge this hypothesis [7,8]. Importantly, the pathogenesis of IVM is still a subject of ongoing research, and more studies are necessary to fully elucidate the mechanisms involved.
IVM of the skull is typically asymptomatic, and neurological deficits are rare. However, the clinical manifestations can vary depending on the lesion’s location and size. As the mass grows, nonspecific headaches may develop. In cases of IVM affecting the frontal and orbital bones, patients may experience proptosis, blepharoptosis, restricted eye movement, and even vision loss. When IVM invades the petrous or sphenoid bones, individuals may present with hearing loss, pulsatile tinnitus, intermittent vertigo, and facial nerve paralysis [6].
Preoperative radiological examinations for cranial IVM typically include plain skull X-rays, CT, and magnetic resonance imaging (MRI). Skull X-ray often reveals an oval or round, well-defined lesion with a “honeycomb” appearance in direct view, or a “sunburst” pattern emanating from a central point in tangential view. CT is particularly crucial as it provides detailed images of the cortical and trabecular bone [4]. Sharma et al. [9] have noted that skull IVMs tend to grow through expansion of the outer table, accompanied by erosion, while the inner table generally remains intact. However, some recent cases have included the invasion and destruction of the inner table [4]. Furthermore, brain MRI is extremely valuable for assessing skull IVMs, as it reveals the depth of the tumor and its relationship with adjacent soft tissues [10].
The definitive diagnosis of IVM can only be established through pathological evaluation following excisional biopsy. This is because even typical radiological features, such as the “sunburst” pattern, require differential diagnosis to exclude other diseases. Skull IVMs must be differentiated from other slow-growing osteolytic lesions, which include calvarial meningioma, Langerhans cell histiocytosis, fibrous dysplasia, osteoma, dermoid cyst, osteogenic sarcoma, and metastasis [4,9].
Treatment approaches for IVM include radiotherapy, curettage, endovascular embolization, and surgical removal. Curettage has fallen out of favor due to the potential risk of intraoperative blood loss and recurrence. En-bloc resection is considered the gold standard for treating IVM, as it not only alleviates the mass effect and achieves a cosmetically satisfactory outcome but also minimizes the risk of bleeding by avoiding disruption of the sinusoids. Preoperative endovascular embolization is recommended to improve the resectability of skull IVMs by reducing their vascularity and firmness [11]. Additionally, endovascular embolization can serve as a palliative treatment for unresectable or inaccessible skull IVMs. For instance, Garcia-Marin et al. [12] described a case of IVM in the occipital condyle that was managed with direct surgical embolization to circumvent the need for condylectomy and instrumental craniocervical stabilization. While radiotherapy may impede tumor growth, it does not reduce tumor volume, and it can lead to scar formation and impaired bone growth in pediatric patients. Radiotherapy therefore is typically reserved for patients with unresectable skull base lesions or residual tumors following subtotal resections [4].
The patient in our case presented with a protruding, slow-growing mass on her forehead that had been present for 10 years. She experienced no symptoms and showed no signs of infection or inflammation; rather, she visited our clinic seeking cosmetic improvement.
In this case, CT revealed trabecular thickening with a sunburst pattern affecting the outer table and causing erosion, while the inner table remained intact. Additionally, the CT scan displayed the characteristic “sunburst” pattern, a typical radiologic feature of skull IVM. We performed en-bloc resection with a margin of approximately 0.5 cm of normal bone, immediately followed by cranioplasty using bone cement. Naama et al. [13] recommend excising IVMs with a 1-cm margin of uninvolved bone to prevent recurrence and significant bleeding. However, in our case, significant bleeding was not encountered, and pathologic findings confirmed that the resection margins were clear.
After en-bloc resection, immediate reconstruction is necessary to ensure cerebral protection, healthy soft tissue coverage, and adequate cosmesis. Reconstructive materials include autologous bone (either vascularized or non-vascularized) and alloplastic mediums such as titanium, polymethylmethacrylate (PMMA), and polyetheretherketone (PEEK) [14-16]. Autologous bone is considered the gold standard for cranioplasty because of its resistance to infection. However, its use is constrained by limited availability, potential donor-site morbidity, and challenges in achieving the desired contour [17]. In contrast, alloplastic implants are widely available, mechanically durable, and easier to shape, but they are more prone to infection and extrusion [15]. In the case presented, cranioplasty was required to address a 4× 4.1-cm bone defect resulting from the en-bloc resection and galeal flap. Given the relatively small nature of the bone defect, we opted to use bone cement for the cranioplasty. Throughout 6 months of follow-up, the patient experienced no postoperative complications. The results were both functionally and aesthetically satisfactory.
Nowadays, with the advancement of various materials, PEEK has been increasingly utilized for cranioplasty, as highlighted previously. Its appeal lies in its strong, thermoplastic properties, resistance to aggressive sterilization, and bone-like elasticity [15,16]. Furthermore, high-resolution CT scans provide data enabling the precise recreation of the defect on a computer-generated model. This allows for the creation of a custom-made (patient-specific) implant that fits the defect exactly [15,16,18]. With such developments, we anticipate achieving both functional and aesthetic outcomes in the removal and reconstruction of skull IVMs, of various sizes and locations.
Notes
Ethical approval
The report was approved by the Institutional Review Board of Hallym University Kangdong Sacred Heart Hospital (IRB No. 2023-08-003).
Fig. 1.
A 59-year-old woman with an intraosseous vascular malformation on the left side of the frontal bone. (A) Preoperative appearance. (B) A photograph after 6 months of en-bloc resection and cranioplasty using bone cement.

Fig. 2.
Preoperative X-ray and three-dimensional computed tomography (CT) scan. (A) Skull X-ray shows a radiolucent osteolytic lesion on the left side of the frontal bone. (B) A coronal view of the CT scan shows a well-circumscribed hyperdense mass upper lateral to the left frontal sinus with a ‘‘sunburst’’ pattern.
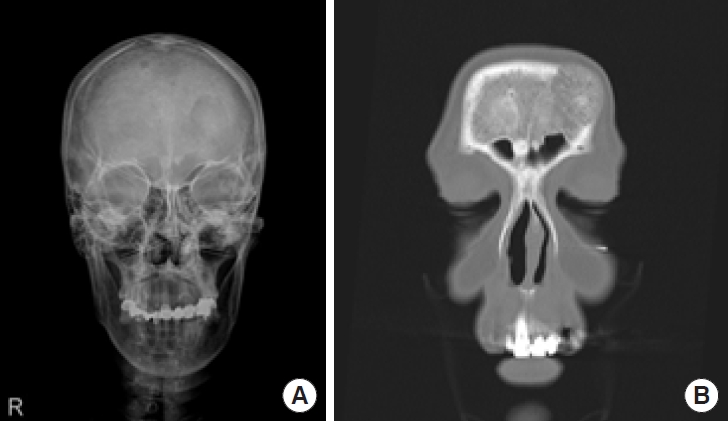
Fig. 3.
Intraoperative photographs. (A) A 3×3.2 cm-sized mass on the left side of the frontal bone. (B) The frontal sinus mucosa was reinforced with a galeal flap. (C) The 4×4.1 cm-sized bone defect was covered with cranioplasty using bone cement.
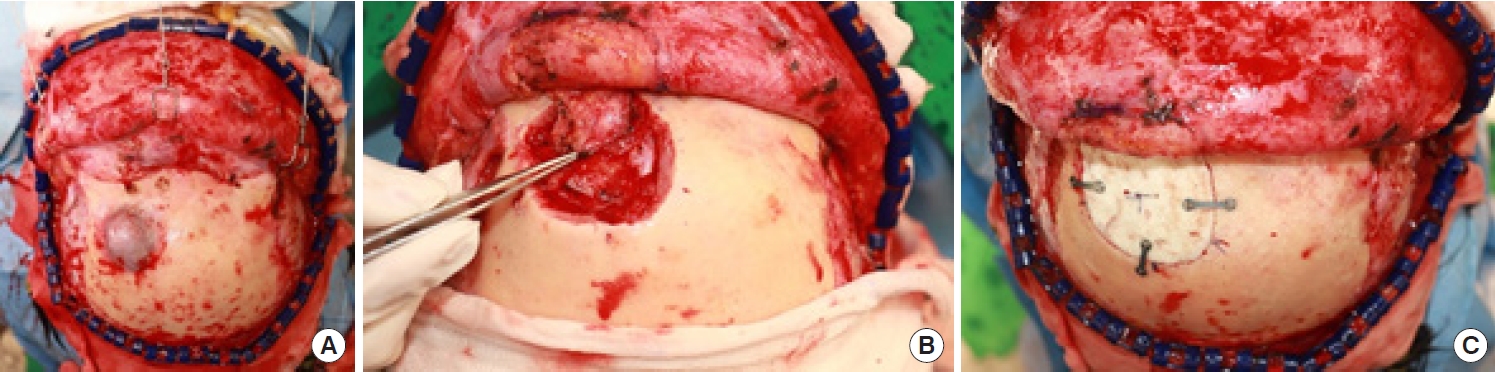
Fig. 4.
Typical pathologic features of the intraosseous vascular malformation show destroyed bony trabeculae (red color *A in this picture) and aberrant loose fibrous stroma (pale bluish color *B). The loose fibrous stroma has many cystic lumina with dilated capillaries and tiny red blood cells as well as expanded stroma (H&E, ×40).

REFERENCES
1. Heckl S, Aschoff A, Kunze S. Cavernomas of the skull: review of the literature 1975-2000. Neurosurg Rev 2002;25:56-67.



2. Toynbee J. An account of two vascular tumors developed in the substance of bone. Lancet 1845;2:676.
4. Wang C, Zhang D, Wang S, Zhang Y, Wang R, Zhao J. Intraosseous cavernous malformations of the skull: clinical characteristics and long-term surgical outcomes. Neurosurg Rev 2020;43:231-9.



5. Vargel I, Cil BE, Er N, Ruacan S, Akarsu AN, Erk Y. Hereditary intraosseous vascular malformation of the craniofacial region: an apparently novel disorder. Am J Med Genet 2002;109:22-35.


6. Yu J, Li Y, Duan X. Posttraumatic cavernous hemangioma of the skull. J Craniofac Surg 2014;25:e48. -51.


7. Chatterji P, Sharma ML, Chatterji S, Kanwar DL. Cavernous haemangioma of fronto-ethmoid region. J Laryngol Otol 1969;83:925-33.


8. Yoshida D, Sugisaki Y, Shimura T, Teramoto A. Cavernous hemangioma of the skull in a neonate. Childs Nerv Syst 1999;15:351-3.



9. Sharma RR, Pawar SJ, Lad SD, Netalkar AS, Musa MM. Frontal intraosseous cryptic hemangioma presenting with supraorbital neuralgia. Clin Neurol Neurosurg 1999;101:215-9.


10. Park BH, Hwang E, Kim CH. Primary intraosseous hemangioma in the frontal bone. Arch Plast Surg 2013;40:283-5.



11. Hishiyama J, Isago T, Ito H. Intraosseous hemangioma of the zygomatic bone. JPRAS Open 2015;6:5-10.

12. Garcia-Marin V, Ravina J, Trujillo E, Gonzalez-Feria L. Symptomatic cavernous hemangioma of the occipital condyle treated with methacrylate embolization. Surg Neurol 2001;56:301-3.


13. Naama O, Gazzaz M, Akhaddar A, Belhachmi A, Asri A, Elmostarchid B, et al. Cavernous hemangioma of the skull: 3 case reports. Surg Neurol 2008;70:654-9.


14. Oliver JD, Banuelos J, Abu-Ghname A, Vyas KS, Sharaf B. Alloplastic cranioplasty reconstruction: a systematic review comparing outcomes with titanium mesh, polymethyl methacrylate, polyether ether ketone, and Norian implants in 3591 adult patients. Ann Plast Surg 2019;82:S289-94.


15. Wang JS, Ter Louw RP, DeFazio MV, McGrail KM, Evans KK. Subtotal calvarial vault reconstruction utilizing a customized polyetheretherketone (PEEK) implant with chimeric microvascular soft tissue coverage in a patient with syndrome of the trephined: a case report. Arch Plast Surg 2019;46:365-70.




16. Moon SJ, Jeon HB, Kim EH, Lew DH, Kim YO, Hong JW. Staged reconstruction of a chronically infected large skull defect using free tissue transfer and a patient-specific polyetheretherketone implant. Arch Craniofac Surg 2020;21:309-14.











