|
 |
- Search
| Arch Craniofac Surg > Volume 24(5); 2023 > Article |
|
Abstract
Notes
Conflict of interest
Jun Ho Choi, Jae Ha Hwang, and Kwang Seog Kim serving as editorial board members of the journal were not involved in the following: selection of the peer reviewer, evaluation of the article, and decision process of acceptance of this article. No other potential conflicts of interest relevant to this article were reported.
Ethical approval
The study was approved by the Institutional Review Board of Chonnam National University Hospital (CNUH-EXP-2023-107).
Patient consent
The patient provided written informed consent for the publication and use of his images.
Author contributions
Conceptualization: Jae Ha Hwang. Data curation: Jun Ho Choi, Soo Hyuk Lee. Formal analysis: Kwang Seog Kim, Sam Yong Lee. Methodology: Kwang Seog Kim, Sam Yong Lee. Project administration: Jae Ha Hwang. Investigation: Jun Ho Choi, Soo Hyuk Lee. Writing - original draft: Jun Ho Choi, Soo Hyuk Lee, Kwang Seog Kim, Jae Ha Hwang, Sam Yong Lee. Writing - review & editing: Jae Ha Hwang.
Fig. 2.

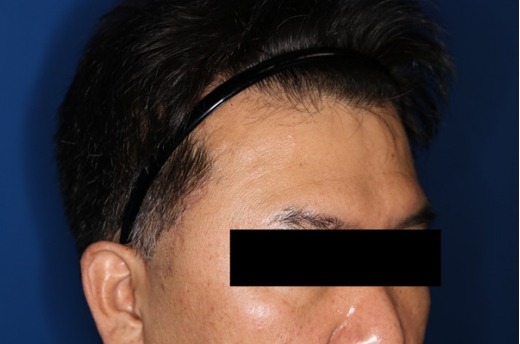
Fig. 4.
Table 1.
| Case report | Age (yr) | Sex | Tumor size (cm) | Site | Treatment | Recurrence or metastasis | Further treatment | Outcome (follow-up) | Immunohistochemistry | Molecular characterization |
|---|---|---|---|---|---|---|---|---|---|---|
| Patel et al. (2013) [8] | 57 | M | 1.5 × 1.5 × 1.3 | Left intraorbital mass (involving lateral rectus muscle) | Excision and lateral orbitotomy | Pleural solitary fibrous tumor (primary) | Stereotactic radiotherapy | Died 3 mo later | (+): CD34, CD99, desmin | NR |
| Lateral left orbit and brain (metastasis) | (–): MYf4, CD31, HMB45, AE1/AE3, S100 | |||||||||
| Dogan et al. (2013) [9] | 27 | F | 2.7 × 2.0 × 1.5 | Left cheek (left masseter muscle) | Excision | NR | NR | 3 yr, no recurrence | (+): CD34, CD99, and Bcl-2, factor XIIIa | NR |
| (–): EMA, pan-cytokeratin, smooth muscle actin, desmin, and S-100 protein | ||||||||||
| Touil et al. (2013) [10] | 36 | F | 5 cm on the long axis | Left occipital region (sternocleidomastoid) | NR | NR | NR | NR | NR | NR |
| Blandamura et al. (2014) [11] | 75 | NR | NR | Right intraorbital mass (involving lateral and medial rectus muscle) | Excision (initial) | 3 Times | Radiotherapy after 1 yr | 3 Relapses with final malignant transformation after 9 yr | (+): CD34, CD99, Bcl-2 | Absence of COL1A1-PDGFB fusion transcripts |
| Chemotherapy after 6 yr | (–): SMA, S100 | |||||||||
| Eyelid-sparing exenteration of the right orbit (final) | ||||||||||
| Current study (2023) | 47 | M | 4.0 × 2.9 × 1.4 | Right temporal region (right temporalis) | Excision | None | None | 8 mo, no recurrence | (+): STAT6, CD99, Bcl-2, actin | NR |
| (–): Desmin, S-100, EMA, CD34 |
REFERENCES
- TOOLS
-
METRICS

-
- 0 Crossref
- Scopus
- 1,007 View
- 33 Download
- Related articles in ACFS
-
Intraosseous vascular malformation of the skull: a case report and literature review ;0()